Whole-brain analytic measures of network communication reveal increased structure-function correlation in right temporal lobe epilepsy
- PMID: 27330970
- PMCID: PMC4909094
- DOI: 10.1016/j.nicl.2016.05.010
Whole-brain analytic measures of network communication reveal increased structure-function correlation in right temporal lobe epilepsy
Abstract
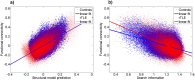
The in vivo structure-function relationship is key to understanding brain network reorganization due to pathologies. This relationship is likely to be particularly complex in brain network diseases such as temporal lobe epilepsy, in which disturbed large-scale systems are involved in both transient electrical events and long-lasting functional and structural impairments. Herein, we estimated this relationship by analyzing the correlation between structural connectivity and functional connectivity in terms of analytical network communication parameters. As such, we targeted the gradual topological structure-function reorganization caused by the pathology not only at the whole brain scale but also both in core and peripheral regions of the brain. We acquired diffusion (dMRI) and resting-state fMRI (rsfMRI) data in seven right-lateralized TLE (rTLE) patients and fourteen healthy controls and analyzed the structure-function relationship by using analytical network communication metrics derived from the structural connectome. In rTLE patients, we found a widespread hypercorrelated functional network. Network communication analysis revealed greater unspecific branching of the shortest path (search information) in the structural connectome and a higher global correlation between the structural and functional connectivity for the patient group. We also found evidence for a preserved structural rich-club in the patient group. In sum, global augmentation of structure-function correlation might be linked to a smaller functional repertoire in rTLE patients, while sparing the central core of the brain which may represent a pathway that facilitates the spread of seizures.
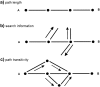
•rTLE patients exhibit increased mean search information compared controls.•Structural search information best predicts functional connectivity in both groups.•Whole brain structure-function correlation is increased in rTLE patients.•Structure-function correlation differs in brain periphery but not in the rich club.
Keywords: CSD, constrained spherical deconvolution; CSF, cerebrospinal fluid; FA, fractional anisotropy; FCA, analytic functional connectivity; FCD, functional connectivity dynamics; FOD, fiber orientation distribution; Functional connectivity; NBS, network based statistics; Network based statistics; Network communication; Rich club; Structural connectivity; Temporal lobe epilepsy; dMRI, diffusion magnetic resonance imaging; rTLE, right temporal lobe epilepsy; rsfMRI, resting state functional magnetic resonance imaging.
Figures
Similar articles
-
Connectivity and tissue microstructural alterations in right and left temporal lobe epilepsy revealed by diffusion spectrum imaging.Neuroimage Clin. 2014 Aug 1;5:349-58. doi: 10.1016/j.nicl.2014.07.013. eCollection 2014. Neuroimage Clin. 2014. PMID: 26236626 Free PMC article.
-
Alteration of functional connectivity within visuospatial working memory-related brain network in patients with right temporal lobe epilepsy: a resting-state fMRI study.Epilepsy Behav. 2014 Jun;35:64-71. doi: 10.1016/j.yebeh.2014.04.001. Epub 2014 May 6. Epilepsy Behav. 2014. PMID: 24810401
-
Alteration of the alertness-related network in patients with right temporal lobe epilepsy: A resting state fMRI study.Epilepsy Res. 2016 Nov;127:252-259. doi: 10.1016/j.eplepsyres.2016.09.013. Epub 2016 Sep 16. Epilepsy Res. 2016. PMID: 27661438
-
Connectome biomarkers of drug-resistant epilepsy.Epilepsia. 2021 Jan;62(1):6-24. doi: 10.1111/epi.16753. Epub 2020 Nov 25. Epilepsia. 2021. PMID: 33236784
-
Short-range connections in the developmental connectome during typical and atypical brain maturation.Neurosci Biobehav Rev. 2017 Dec;83:109-122. doi: 10.1016/j.neubiorev.2017.10.007. Epub 2017 Oct 9. Neurosci Biobehav Rev. 2017. PMID: 29024679 Free PMC article. Review.
Cited by
-
Differences in MEG and EEG power-law scaling explained by a coupling between spatial coherence and frequency: a simulation study.J Comput Neurosci. 2019 Aug;47(1):31-41. doi: 10.1007/s10827-019-00721-9. Epub 2019 Jul 11. J Comput Neurosci. 2019. PMID: 31292816
-
Dynamical Mechanisms of Interictal Resting-State Functional Connectivity in Epilepsy.J Neurosci. 2020 Jul 15;40(29):5572-5588. doi: 10.1523/JNEUROSCI.0905-19.2020. Epub 2020 Jun 8. J Neurosci. 2020. PMID: 32513827 Free PMC article.
-
Mapping the structural and functional network architecture of the medial temporal lobe using 7T MRI.Hum Brain Mapp. 2018 Feb;39(2):851-865. doi: 10.1002/hbm.23887. Epub 2017 Nov 20. Hum Brain Mapp. 2018. PMID: 29159960 Free PMC article.
-
Functional and structural connectivity substrates of cognitive performance in relapsing remitting multiple sclerosis with mild disability.Neuroimage Clin. 2020;25:102177. doi: 10.1016/j.nicl.2020.102177. Epub 2020 Jan 12. Neuroimage Clin. 2020. PMID: 32014828 Free PMC article.
-
Altered correlation of concurrently recorded EEG-fMRI connectomes in temporal lobe epilepsy.Netw Neurosci. 2024 Jul 1;8(2):466-485. doi: 10.1162/netn_a_00362. eCollection 2024. Netw Neurosci. 2024. PMID: 38952816 Free PMC article.
References
-
- Arthuis M., Valton L., Regis J., Chauvel P., Wendling F., Naccache L., Bernard C., Bartolomei F. Impaired consciousness during temporal lobe seizures is related to increased long-distance cortical-subcortical synchronization. Brain. 2009;132:2091–2101. - PubMed
-
- Avanzini G., Depaulis A., Tassinari A., de Curtis M. Do seizures and epileptic activity worsen epilepsy and deteriorate cognitive function? Epilepsia. 2013;54(Suppl. 8):14–21. - PubMed
-
- Bartolomei F., Wendling F., Bellanger J.J., Regis J., Chauvel P. Neural networks involving the medial temporal structures in temporal lobe epilepsy. Clin. Neurophysiol. 2001;112:1746–1760. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

