The Effect of Covalently-Attached ATRP-Synthesized Polymers on Membrane Stability and Cytoprotection in Human Erythrocytes
- PMID: 27331401
- PMCID: PMC4917246
- DOI: 10.1371/journal.pone.0157641
The Effect of Covalently-Attached ATRP-Synthesized Polymers on Membrane Stability and Cytoprotection in Human Erythrocytes
Abstract
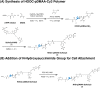
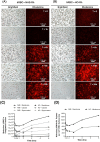
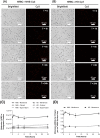
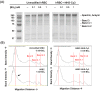
Erythrocytes have been described as advantageous drug delivery vehicles. In order to ensure an adequate circulation half-life, erythrocytes may benefit from protective enhancements that maintain membrane integrity and neutralize oxidative damage of membrane proteins that otherwise facilitate their premature clearance from circulation. Surface modification of erythrocytes using rationally designed polymers, synthesized via atom-transfer radical polymerization (ATRP), may further expand the field of membrane-engineered red blood cells. This study describes the fate of ATRP-synthesized polymers that were covalently attached to human erythrocytes as well as the effect of membrane engineering on cell stability under physiological and oxidative conditions in vitro. The biocompatible, membrane-reactive polymers were homogenously retained on the periphery of modified erythrocytes for at least 24 hours. Membrane engineering stabilized the erythrocyte membrane and effectively neutralized oxidative species, even in the absence of free-radical scavenger-containing polymers. The targeted functionalization of Band 3 protein by NHS-pDMAA-Cy3 polymers stabilized its monomeric form preventing aggregation in the presence of the crosslinking reagent, bis(sulfosuccinimidyl)suberate (BS3). A free radical scavenging polymer, NHS-pDMAA-TEMPO˙, provided additional protection of surface modified erythrocytes in an in vitro model of oxidative stress. Preserving or augmenting cytoprotective mechanisms that extend circulation half-life is an important consideration for the use of red blood cells for drug delivery in various pathologies, as they are likely to encounter areas of imbalanced oxidative stress as they circuit the vascular system.
Conflict of interest statement
Figures



References
-
- Siems WG, Sommerburg O, Grune T. Erythrocyte free radical and energy metabolism. Clinical nephrology. 2000;53(1 Suppl):S9–17. - PubMed
-
- Szweda-Lewandowska Z, Krokosz A, Gonciarz M, Zajeczkowska W, Puchala M. Damage to human erythrocytes by radiation-generated HO* radicals: molecular changes in erythrocyte membranes. Free radical research. 2003;37(10):1137–43. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

