RRD-251 enhances all-trans retinoic acid (RA)-induced differentiation of HL-60 myeloblastic leukemia cells
- PMID: 27331409
- PMCID: PMC5216806
- DOI: 10.18632/oncotarget.10136
RRD-251 enhances all-trans retinoic acid (RA)-induced differentiation of HL-60 myeloblastic leukemia cells
Abstract
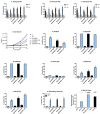
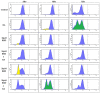
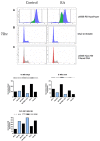
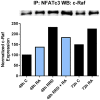
All-trans-retinoic acid (RA) is known to induce terminal granulocytic differentiation and cell cycle arrest of HL-60 cells. Responding to an RA-induced cytosolic signaling machine, c-Raf translocates to the nucleus, providing propulsion for RA-induced differentiation. This novel mechanism is not understood, but presumably reflects c-Raf binding with nuclear gene regulatory proteins. RRD-251 is a small molecule that prevents the interaction of c-Raf and RB, the retinoblastoma tumor suppressor protein. The involvement of c-Raf and RB in RA-induced differentiation motivates interest in the effects of combined RA and RRD-251 treatment on leukemic cell differentiation. We demonstrate that RRD-251 enhances RA-induced differentiation. Mechanistically, we find that nuclear translocated c-Raf associates with pS608 RB. RA causes loss of pS608 RB, where cells with hypophosphorylated S608 RB are G0/G1 restricted. Corroborating the pS608 RB hypophosphorylation, RB sequestration of E2F increased with concomitant loss of cdc6 expression, which is known to be driven by E2F. Hypophosphorylation of S608 RB releases c-Raf from RB sequestration to bind other nuclear targets. Release of c-Raf from RB sequestration results in enhanced association with GSK-3 which is phosphorylated at its S21/9 inhibitory sites. c-Raf binding to GSK-3 is associated with dissociation of GSK-3 and RARα, thereby relieving RARα of GSK-3 inhibition. RRD-251 amplifies each of these RA-induced events. Consistent with the posited enhancement of RARα transcriptional activity by RRD-251, RRD-251 increases the RARE-driven CD38 expression per cell. The RA/c-Raf/GSK-3/RARα axis emerges as a novel differentiation regulatory mechanism susceptible to RRD-251, suggesting enhancing RA-effects with RRD-251 in therapy.
Keywords: GSK-3; RRD-251; c-Raf; retinoblastoma protein (RB); all-trans retinoic acid (RA).
Conflict of interest statement
None of the authors have competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

