Micromechanical Analysis of the Hyaluronan-Rich Matrix Surrounding the Oocyte Reveals a Uniquely Soft and Elastic Composition
- PMID: 27332136
- PMCID: PMC4919725
- DOI: 10.1016/j.bpj.2016.03.023
Micromechanical Analysis of the Hyaluronan-Rich Matrix Surrounding the Oocyte Reveals a Uniquely Soft and Elastic Composition
Abstract
The cumulus cell-oocyte complex (COC) matrix is an extended coat that forms around the oocyte a few hours before ovulation and plays vital roles in oocyte biology. Here, we analyzed the micromechanical response of mouse COC matrix by colloidal-probe atomic force microscopy. We found that the COC matrix is elastic insofar as it does not flow and its original shape is restored after force release. At the same time, the COC matrix is extremely soft. Specifically, the most compliant parts of in vivo and in vitro expanded COC matrices yielded Young's modulus values of 0.5 ± 0.1 Pa and 1.6 ± 0.3 Pa, respectively, suggesting both high porosity and a large mesh size (≥100 nm). In addition, the elastic modulus increased progressively with indentation. Furthermore, using optical microscopy to correlate these mechanical properties with ultrastructure, we discovered that the COC is surrounded by a thick matrix shell that is essentially devoid of cumulus cells and is enhanced upon COC expansion in vivo. We propose that the pronounced nonlinear elastic behavior of the COC matrix is a consequence of structural heterogeneity and serves important functions in biological processes such as oocyte transport in the oviduct and sperm penetration.
Copyright © 2016 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
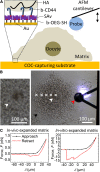

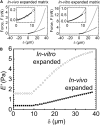
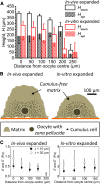
Comment in
-
The Mechanics of Ovulation Depend on an Incredibly Soft and Sugar-Rich Extracellular Matrix.Biophys J. 2016 Jun 21;110(12):2566-2567. doi: 10.1016/j.bpj.2016.04.049. Biophys J. 2016. PMID: 27332115 Free PMC article. No abstract available.
References
-
- Russell D.L., Salustri A. Extracellular matrix of the cumulus-oocyte complex. Semin. Reprod. Med. 2006;24:217–227. - PubMed
-
- Salustri A., Yanagishita M., Hascall V.C. Localization and synthesis of hyaluronic acid in the cumulus cells and mural granulosa cells of the preovulatory follicle. Dev. Biol. 1992;151:541–551. - PubMed
-
- Chen L., Mao S.J., Larsen W.J. Identification of a factor in fetal bovine serum that stabilizes the cumulus extracellular matrix. A role for a member of the inter-alpha-trypsin inhibitor family. J. Biol. Chem. 1992;267:12380–12386. - PubMed
-
- Salustri A., Garlanda C., Mantovani A. PTX3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in in vivo fertilization. Development. 2004;131:1577–1586. - PubMed
-
- Fülöp C., Szántó S., Mikecz K. Impaired cumulus mucification and female sterility in tumor necrosis factor-induced protein-6 deficient mice. Development. 2003;130:2253–2261. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

