Abdominal manifestations of histiocytic disorders in adults: imaging perspective
- PMID: 27332519
- PMCID: PMC5124924
- DOI: 10.1259/bjr.20160221
Abdominal manifestations of histiocytic disorders in adults: imaging perspective
Abstract
Histiocytic disorders (HDs) are a diverse group of diseases characterized by pathologic infiltration of normal tissues by cells of the mononuclear phagocyte system. The spectrum of these diseases ranges from treatable infectious diseases to rapidly progressive, life-threatening conditions. Although they are rare and difficult diagnoses, HDs can be diagnosed with the help of clinical and laboratory analyses, imaging features and tissue biopsy. The clinicopathology and imaging spectrum of select entities belonging to this disorder are presented in this review.
Figures
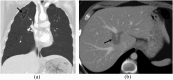
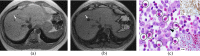
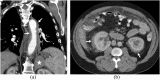
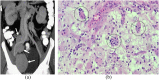
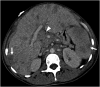
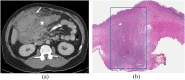
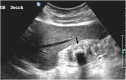

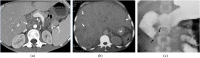
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

