State Legal Restrictions and Prescription-Opioid Use among Disabled Adults
- PMID: 27332619
- PMCID: PMC4985562
- DOI: 10.1056/NEJMsa1514387
State Legal Restrictions and Prescription-Opioid Use among Disabled Adults
Abstract
Background: In response to rising rates of opioid abuse and overdose, U.S. states enacted laws to restrict the prescribing and dispensing of controlled substances. The effect of these laws on opioid use is unclear.
Methods: We tested associations between prescription-opioid receipt and state controlled-substances laws. Using Medicare administrative data for fee-for-service disabled beneficiaries 21 to 64 years of age who were alive throughout the calendar year (8.7 million person-years from 2006 through 2012) and an original data set of laws (e.g., prescription-drug monitoring programs), we examined the annual prevalence of beneficiaries with four or more opioid prescribers, prescriptions yielding a daily morphine-equivalent dose (MED) of more than 120 mg, and treatment for nonfatal prescription-opioid overdose. We estimated how opioid outcomes varied according to eight types of laws.
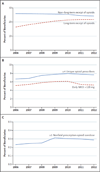
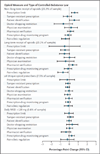
Results: From 2006 through 2012, states added 81 controlled-substance laws. Opioid receipt and potentially hazardous prescription patterns were common. In 2012 alone, 47% of beneficiaries filled opioid prescriptions (25% in one to three calendar quarters and 22% in every calendar quarter); 8% had four or more opioid prescribers; 5% had prescriptions yielding a daily MED of more than 120 mg in any calendar quarter; and 0.3% were treated for a nonfatal prescription-opioid overdose. We observed no significant associations between opioid outcomes and specific types of laws or the number of types enacted. For example, the percentage of beneficiaries with a prescription yielding a daily MED of more than 120 mg did not decline after adoption of a prescription-drug monitoring program (0.27 percentage points; 95% confidence interval, -0.05 to 0.59).
Conclusions: Adoption of controlled-substance laws was not associated with reductions in potentially hazardous use of opioids or overdose among disabled Medicare beneficiaries, a population particularly at risk. (Funded by the National Institute on Aging and others.).
Figures


Comment in
-
State Regulations and Opioid Use among Disabled Adults.N Engl J Med. 2016 Oct 6;375(14):1397. doi: 10.1056/NEJMc1610108. N Engl J Med. 2016. PMID: 27705247 No abstract available.
-
State Regulations and Opioid Use among Disabled Adults.N Engl J Med. 2016 Oct 6;375(14):1396-1397. doi: 10.1056/NEJMc1610108. N Engl J Med. 2016. PMID: 27705248 No abstract available.
References
-
- Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372:241–248. - PubMed
-
- Rutkow L, Chang HY, Daubresse M, Webster DW, Stuart EA, Alexander GC. Effect of Florida’s prescription drug monitoring program and pill mill laws on opioid prescribing and use. JAMA Intern Med. 2015;175:1642–1649. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
