Adapted Treatment Guided by Interim PET-CT Scan in Advanced Hodgkin's Lymphoma
- PMID: 27332902
- PMCID: PMC4961236
- DOI: 10.1056/NEJMoa1510093
Adapted Treatment Guided by Interim PET-CT Scan in Advanced Hodgkin's Lymphoma
Abstract
Background: We tested interim positron-emission tomography-computed tomography (PET-CT) as a measure of early response to chemotherapy in order to guide treatment for patients with advanced Hodgkin's lymphoma.
Methods: Patients with newly diagnosed advanced classic Hodgkin's lymphoma underwent a baseline PET-CT scan, received two cycles of ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) chemotherapy, and then underwent an interim PET-CT scan. Images were centrally reviewed with the use of a 5-point scale for PET findings. Patients with negative PET findings after two cycles were randomly assigned to continue ABVD (ABVD group) or omit bleomycin (AVD group) in cycles 3 through 6. Those with positive PET findings after two cycles received BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone). Radiotherapy was not recommended for patients with negative findings on interim scans. The primary outcome was the difference in the 3-year progression-free survival rate between randomized groups, a noninferiority comparison to exclude a difference of 5 or more percentage points.
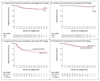
Results: A total of 1214 patients were registered; 937 of the 1119 patients (83.7%) who underwent an interim PET-CT scan according to protocol had negative findings. With a median follow-up of 41 months, the 3-year progression-free survival rate and overall survival rate in the ABVD group were 85.7% (95% confidence interval [CI], 82.1 to 88.6) and 97.2% (95% CI, 95.1 to 98.4), respectively; the corresponding rates in the AVD group were 84.4% (95% CI, 80.7 to 87.5) and 97.6% (95% CI, 95.6 to 98.7). The absolute difference in the 3-year progression-free survival rate (ABVD minus AVD) was 1.6 percentage points (95% CI, -3.2 to 5.3). Respiratory adverse events were more severe in the ABVD group than in the AVD group. BEACOPP was given to the 172 patients with positive findings on the interim scan, and 74.4% had negative findings on a third PET-CT scan; the 3-year progression-free survival rate was 67.5% and the overall survival rate 87.8%. A total of 62 patients died during the trial (24 from Hodgkin's lymphoma), for a 3-year progression-free survival rate of 82.6% and an overall survival rate of 95.8%.
Conclusions: Although the results fall just short of the specified noninferiority margin, the omission of bleomycin from the ABVD regimen after negative findings on interim PET resulted in a lower incidence of pulmonary toxic effects than with continued ABVD but not significantly lower efficacy. (Funded by Cancer Research UK and Others; ClinicalTrials.gov number, NCT00678327.).
Figures

Comment in
-
Fine-Tuning the Treatment of Hodgkin's Lymphoma.N Engl J Med. 2016 Jun 23;374(25):2490-2. doi: 10.1056/NEJMe1604026. N Engl J Med. 2016. PMID: 27332908 No abstract available.
-
Imaging: Follow your PET for guidance.Nat Rev Clin Oncol. 2016 Aug;13(8):463. doi: 10.1038/nrclinonc.2016.110. Epub 2016 Jul 12. Nat Rev Clin Oncol. 2016. PMID: 27402575 No abstract available.
-
Interim PET-CT Scan in Advanced Hodgkin's Lymphoma.N Engl J Med. 2016 Sep 8;375(10):999-1000. doi: 10.1056/NEJMc1609333. N Engl J Med. 2016. PMID: 27602676 No abstract available.
-
Interim PET-CT Scan in Advanced Hodgkin's Lymphoma.N Engl J Med. 2016 Sep 8;375(10):999. doi: 10.1056/NEJMc1609333. N Engl J Med. 2016. PMID: 27602677 No abstract available.
-
Modifying therapy in patients with advanced Hodgkin's lymphoma by integrating early metabolic response by interim PET-CT.Ann Transl Med. 2016 Oct;4(Suppl 1):S19. doi: 10.21037/atm.2016.10.20. Ann Transl Med. 2016. PMID: 27867987 Free PMC article. No abstract available.
-
Advanced Hodgkin's lymphoma: End-of-treatment FDG-PET should be maintained.Eur J Nucl Med Mol Imaging. 2017 Aug;44(8):1254-1257. doi: 10.1007/s00259-017-3714-4. Eur J Nucl Med Mol Imaging. 2017. PMID: 28466283 No abstract available.
References
-
- Bonadonna G, Zucali R, Monfardini S, De Lena M, Uslenghi C. Combination chemotherapy of Hodgkin’s disease with adriamycin, bleomycin, vinblastine, and imidazole carboxamide versus MOPP. Cancer. 1975;36:252–9. - PubMed
-
- Canellos GP, Anderson JR, Propert KJ, et al. Chemotherapy of advanced Hodgkin’s disease with MOPP, ABVD, or MOPP alternating with ABVD. N Engl J Med. 1992;327:1478–84. - PubMed
-
- Viviani S, Bonadonna G, Santoro A, et al. Alternating versus hybrid MOPP and ABVD combinations in advanced Hodgkin’s disease: ten-year results. J Clin Oncol. 1996;14:1421–30. - PubMed
-
- Duggan DB, Petroni GR, Johnson JL, et al. Randomized comparison of ABVD and MOPP/ABV hybrid for the treatment of advanced Hodgkin’s disease: report of an intergroup trial. J Clin Oncol. 2003;21:607–14. - PubMed
-
- Johnson PWM, Radford JA, Cullen MH, et al. Comparison of ABVD and alternating or hybrid multidrug regimens for the treatment of advanced Hodgkin’s lymphoma: results of the United Kingdom Lymphoma Group LY09 Trial (ISRCTN97144519) J Clin Oncol. 2005;23:9208–18. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
