Upregulation of Haploinsufficient Gene Expression in the Brain by Targeting a Long Non-coding RNA Improves Seizure Phenotype in a Model of Dravet Syndrome
- PMID: 27333023
- PMCID: PMC4972487
- DOI: 10.1016/j.ebiom.2016.05.011
Upregulation of Haploinsufficient Gene Expression in the Brain by Targeting a Long Non-coding RNA Improves Seizure Phenotype in a Model of Dravet Syndrome
Abstract
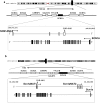
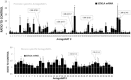
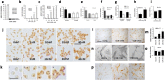
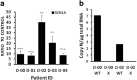
Dravet syndrome is a devastating genetic brain disorder caused by heterozygous loss-of-function mutation in the voltage-gated sodium channel gene SCN1A. There are currently no treatments, but the upregulation of SCN1A healthy allele represents an appealing therapeutic strategy. In this study we identified a novel, evolutionary conserved mechanism controlling the expression of SCN1A that is mediated by an antisense non-coding RNA (SCN1ANAT). Using oligonucleotide-based compounds (AntagoNATs) targeting SCN1ANAT we were able to induce specific upregulation of SCN1A both in vitro and in vivo, in the brain of Dravet knock-in mouse model and a non-human primate. AntagoNAT-mediated upregulation of Scn1a in postnatal Dravet mice led to significant improvements in seizure phenotype and excitability of hippocampal interneurons. These results further elucidate the pathophysiology of Dravet syndrome and outline a possible new approach for the treatment of this and other genetic disorders with similar etiology.
Keywords: AntagoNAT; Dravet syndrome; Long non-coding RNA; Natural antisense transcript; Oligonucleotide-based compound; SCN1A.
Copyright © 2016 The Authors. Published by Elsevier B.V. All rights reserved.
Figures



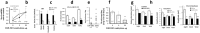

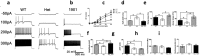

References
-
- Bechi G., Scalmani P., Schiavon E., Rusconi R., Franceschetti S., Mantegazza M. Pure halopinsufficiency for Dravet syndrome Nav1.1 (SCN1A) sodium channel truncating mutations. Epilepsia. 2011 (1528-1167 PMID: 22150645) - PubMed
-
- BrainSpan Atlas http://www.brainspan.org (accessed 05/02/2016)
-
- Cahoy J.D., Emery B., Kaushal A., Foo L.C., Zamanian J.L., Christopherson K.S., Xing Y., Lubischer J.L., Krieg P.A., Krupenko S.A., Thompson W.J., Barres B.A. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J. Neurosci. 2008;28(1):264–278. (Jan 2) - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

