Neurocognition with maraviroc compared with tenofovir in HIV
- PMID: 27333088
- PMCID: PMC5014739
- DOI: 10.1097/QAD.0000000000001189
Neurocognition with maraviroc compared with tenofovir in HIV
Abstract
Objective: The objective was to determine whether maraviroc (MVC) has unique neurocognitive benefits in the context of initial antiretroviral therapy (ART).
Design: Randomized, double-blind, placebo-controlled, 48-week trial.
Setting: Participants were enrolled in US AIDS Clinical Trials Group clinical trial sites.
Participants: Total 262 ART-naive, chemokine coreceptor 5 tropic HIV, and HIV RNA greater than 1000 copies/ml participants were randomized, 230 participants completed the study.
Intervention: Participants received MVC 150 mg or tenofovir disoproxil fumarate (TDF) 300 mg on a background of ritonavir-boosted darunavir and emtricitabine.
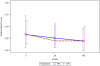
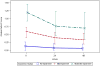
Main outcome measure(s): The neuropsychological battery of 15 tests done at baseline, week 24 and week 48 assessed seven domains, and were standardized into z-scores then converted into deficit scores and a global deficit score. The 48-week changes from baseline in the neuropsychological scores and the global deficit score were compared by Wilcoxon or Kruskal-Wallis test between arms, and among baseline impairment groups [classified as normal, mild (2 deficit scores ≥1) and moderate (2 deficit scores ≥2)]. It was hypothesized that the MVC arm would have improved neuropsychological performance over TDF.
Results: In this double-blind, randomized, placebo-controlled trial, there were no differences in neuropsychological performance between MVC and TDF. Those with moderate neuropsychological impairment at baseline experienced greater ART-mediated neuropsychological improvement than those with mild or no neuropsychological impairment.
Conclusion: Improvement in neurocognitive functioning was greater with more baseline impairment but was comparable with MVC or TDF.
Conflict of interest statement
For the remaining authors no conflicts were declared.
Figures
References
-
- Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, et al. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS. 2007;21(14):1915–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical