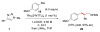Enantioselective Intermolecular C-H Functionalization of Allylic and Benzylic sp(3) C-H Bonds Using N-Sulfonyl-1,2,3-triazoles
- PMID: 27333162
- PMCID: PMC5521008
- DOI: 10.1021/acs.orglett.6b01298
Enantioselective Intermolecular C-H Functionalization of Allylic and Benzylic sp(3) C-H Bonds Using N-Sulfonyl-1,2,3-triazoles
Abstract
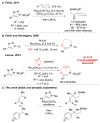
The enantioselective intermolecular sp(3) C-H functionalization at the allylic and benzylic positions was achieved using rhodium-catalyzed reactions with 4-phenyl-N-(methanesulfonyl)-1,2,3-triazole. The optimum dirhodium tetracarboxylate catalyst for these reactions was Rh2(S-NTTL)4. The rhodium-bound α-imino carbene intermediates preferentially reacted with tertiary over primary C-H bonds in good yields and moderate levels of enantioselectivity (66-82% ee). This work demonstrates that N-sulfonyltriazoles can be applied to the effective C-H functionalization at sp(3) C-H bonds of substrates containing additional functionality.
Figures
References
-
-
For an overview of recent advances in C–H functionalization, see: Davies HML, Morton D. J Org Chem. 2016;81:343–350.Farmer ME, Laforteza BN, Yu JQ. Bioorg Med Chem. 2014;22:4445–4452.Brückl T, Baxter RD, Ishihara Y, Baran PS. Acc Chem Res. 2012;45:826–839.Gutekunst WR, Baran PS. Chem Soc Rev. 2011;40:1976–1991.Newhouse T, Baran PS. Angew Chem Int Ed. 2011;50:3362–3374.
-
-
-
For C–H functionalization for materials and natural products, see: McMurray L, O’Hara F, Gaunt MJ. Chem Soc Rev. 2011;40:1885–1898.Yamaguchi J, Yamaguchi AD, Itami K. Angew Chem Int Ed. 2012;51:8960–9009.Ku-ninobu Y, Sueki S. Synthesis. 2015;47:3823–3845.
-
-
-
For a review on the use of C–H functionalization in medicinal chemistry, see: Cernak T, Dykstra KD, Tyagarajan S, Vachal P, Krska SW. Chem Soc Rev. 2016;45:546–576.
-
-
-
For recent methods for C–H functionalization using Rh(I)-Rh(III) catalytic cycle review, see: Motevalli S, Sokeirik Y, Ghanem A. Eur J Org Chem. 2016;8:1459–1475.
-
-
-
For reviews on C–H functionalization using donor-acceptor metalocarbenes, see: Davies HML, Manning JR. Nature. 2008;451:417–424.Davies HML, Morton D. Chem Soc Rev. 2011;40:1857–1869.Davies HML, Lian Y. Acc Chem Res. 2012;45:923–935.. For recent examples of C–H functionalization using donor-acceptor metalocarbenes, see: Qin C, Davies HML. J Am Chem Soc. 2014;136:9792.Guptill DM, Davies HML. J Am Chem Soc. 2014;136:17718–17721.Fu L, Guptill DM, Davies HML. J Am Chem Soc. 2016;138:5761–5764.Liao K, Negretti S, Musaev DG, Bacsa J, Davies HML. Nature. 2016;533:230–234.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources