Synaptopathies: synaptic dysfunction in neurological disorders - A review from students to students
- PMID: 27333343
- PMCID: PMC5095804
- DOI: 10.1111/jnc.13713
Synaptopathies: synaptic dysfunction in neurological disorders - A review from students to students
Abstract
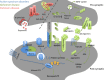
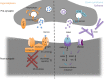
Synapses are essential components of neurons and allow information to travel coordinately throughout the nervous system to adjust behavior to environmental stimuli and to control body functions, memories, and emotions. Thus, optimal synaptic communication is required for proper brain physiology, and slight perturbations of synapse function can lead to brain disorders. In fact, increasing evidence has demonstrated the relevance of synapse dysfunction as a major determinant of many neurological diseases. This notion has led to the concept of synaptopathies as brain diseases with synapse defects as shared pathogenic features. In this review, which was initiated at the 13th International Society for Neurochemistry Advanced School, we discuss basic concepts of synapse structure and function, and provide a critical view of how aberrant synapse physiology may contribute to neurodevelopmental disorders (autism, Down syndrome, startle disease, and epilepsy) as well as neurodegenerative disorders (Alzheimer and Parkinson disease). We finally discuss the appropriateness and potential implications of gathering synapse diseases under a single term. Understanding common causes and intrinsic differences in disease-associated synaptic dysfunction could offer novel clues toward synapse-based therapeutic intervention for neurological and neuropsychiatric disorders. In this Review, which was initiated at the 13th International Society for Neurochemistry (ISN) Advanced School, we discuss basic concepts of synapse structure and function, and provide a critical view of how aberrant synapse physiology may contribute to neurodevelopmental (autism, Down syndrome, startle disease, and epilepsy) as well as neurodegenerative disorders (Alzheimer's and Parkinson's diseases), gathered together under the term of synaptopathies. Read the Editorial Highlight for this article on page 783.
Keywords: Alzheimer disease; Down syndrome; autism; epilepsy; hyperekplexia; synapses.
© 2016 The Authors. Journal of Neurochemistry published by John Wiley & Sons Ltd on behalf of International Society for Neurochemistry.
Figures
References
-
- Agarwala K. L., Ganesh S., Suzuki T. et al (2001) Dscam is associated with axonal and dendritic features of neuronal cells. J. Neurosci. Res. 66, 337–346. - PubMed
-
- Al‐Owain M., Colak D., Al‐Bakheet A. et al (2012) Novel mutation in GLRB in a large family with hereditary hyperekplexia. Clin. Genet. 81, 479–484. - PubMed
-
- Andermann F., Keene D., Andermann E. and Quesney L. (1980) Startle disease or hyperekplexia: further delineation of the syndrome. Brain 103, 985–997. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

