Human Skin Constructs with Spatially Controlled Vasculature Using Primary and iPSC-Derived Endothelial Cells
- PMID: 27333469
- PMCID: PMC5031081
- DOI: 10.1002/adhm.201500936
Human Skin Constructs with Spatially Controlled Vasculature Using Primary and iPSC-Derived Endothelial Cells
Abstract
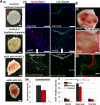
Vascularization of engineered human skin constructs is crucial for recapitulation of systemic drug delivery and for their long-term survival, functionality, and viable engraftment. In this study, the latest microfabrication techniques are used and a novel bioengineering approach is established to micropattern spatially controlled and perfusable vascular networks in 3D human skin equivalents using both primary and induced pluripotent stem cell (iPSC)-derived endothelial cells. Using 3D printing technology makes it possible to control the geometry of the micropatterned vascular networks. It is verified that vascularized human skin equivalents (vHSEs) can form a robust epidermis and establish an endothelial barrier function, which allows for the recapitulation of both topical and systemic delivery of drugs. In addition, the therapeutic potential of vHSEs for cutaneous wounds on immunodeficient mice is examined and it is demonstrated that vHSEs can both promote and guide neovascularization during wound healing. Overall, this innovative bioengineering approach can enable in vitro evaluation of topical and systemic drug delivery as well as improve the potential of engineered skin constructs to be used as a potential therapeutic option for the treatment of cutaneous wounds.
Keywords: engineered skin; iPSC; microfluidics; patterning; vasculature.
© 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

