A Randomized Controlled Trial to Prevent Depression and Ameliorate Insulin Resistance in Adolescent Girls at Risk for Type 2 Diabetes
- PMID: 27333897
- PMCID: PMC5055426
- DOI: 10.1007/s12160-016-9801-0
A Randomized Controlled Trial to Prevent Depression and Ameliorate Insulin Resistance in Adolescent Girls at Risk for Type 2 Diabetes
Abstract
Background: Prospective data suggest depressive symptoms worsen insulin resistance and accelerate type 2 diabetes (T2D) onset.
Purpose: We sought to determine whether reducing depressive symptoms in overweight/obese adolescents at risk for T2D would increase insulin sensitivity and mitigate T2D risk.
Method: We conducted a parallel-group, randomized controlled trial comparing a 6-week cognitive-behavioral (CB) depression prevention group with a 6-week health education (HE) control group in 119 overweight/obese adolescent girls with mild-to-moderate depressive symptoms (Center for Epidemiological Studies-Depression Scale [CES-D] ≥16) and T2D family history. Primary outcomes were baseline to post-intervention changes in CES-D and whole body insulin sensitivity index (WBISI), derived from 2-h oral glucose tolerance tests. Outcome changes were compared between groups using ANCOVA, adjusting for respective baseline outcome, puberty, race, facilitator, T2D family history degree, baseline age, adiposity, and adiposity change. Multiple imputation was used for missing data.
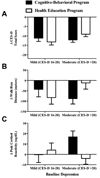
Results: Depressive symptoms decreased (p < 0.001) in CB and HE from baseline to posttreatment, but did not differ between groups (ΔCESD = -12 vs. -11, 95 % CI difference = -4 to +1, p = 0.31). Insulin sensitivity was stable (p > 0.29) in CB and HE (ΔWBISI = 0.1 vs. 0.2, 95 % CI difference = -0.6 to +0.4, p = 0.63). Among all participants, reductions in depressive symptoms were associated with improvements in insulin sensitivity (p = 0.02).
Conclusions: Girls at risk for T2D displayed reduced depressive symptoms following 6 weeks of CB or HE. Decreases in depressive symptoms related to improvements in insulin sensitivity. Longer-term follow-up is needed to determine whether either program causes sustained decreases in depressive symptoms and improvements in insulin sensitivity.
Trial registration number: The trial was registered with clinicaltrials.gov (NCT01425905).
Keywords: Adolescence; Depression; Insulin resistance; Randomized controlled trial; Type 2 diabetes.
Conflict of interest statement
The authors having nothing to disclose.
Figures


References
-
- Gregg EW, Zhuo X, Cheng YJ, et al. Trends in lifetime risk and years of life lost due to diabetes in the USA, 1985–2011: a modelling study. Lancet Diabetes Endocrinology. 2014;2:867–874. - PubMed
-
- Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24:1069–1078. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

