Complement is activated in progressive multiple sclerosis cortical grey matter lesions
- PMID: 27333900
- PMCID: PMC4918026
- DOI: 10.1186/s12974-016-0611-x
Complement is activated in progressive multiple sclerosis cortical grey matter lesions
Abstract
Background: The symptoms of multiple sclerosis (MS) are caused by damage to myelin and nerve cells in the brain and spinal cord. Inflammation is tightly linked with neurodegeneration, and it is the accumulation of neurodegeneration that underlies increasing neurological disability in progressive MS. Determining pathological mechanisms at play in MS grey matter is therefore a key to our understanding of disease progression.
Methods: We analysed complement expression and activation by immunocytochemistry and in situ hybridisation in frozen or formalin-fixed paraffin-embedded post-mortem tissue blocks from 22 progressive MS cases and made comparisons to inflammatory central nervous system disease and non-neurological disease controls.
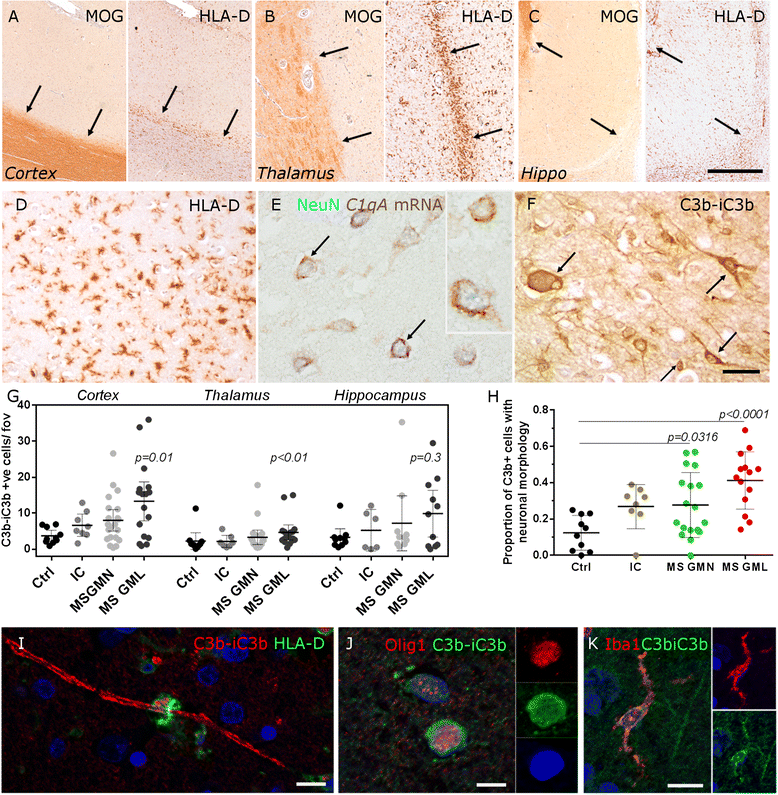
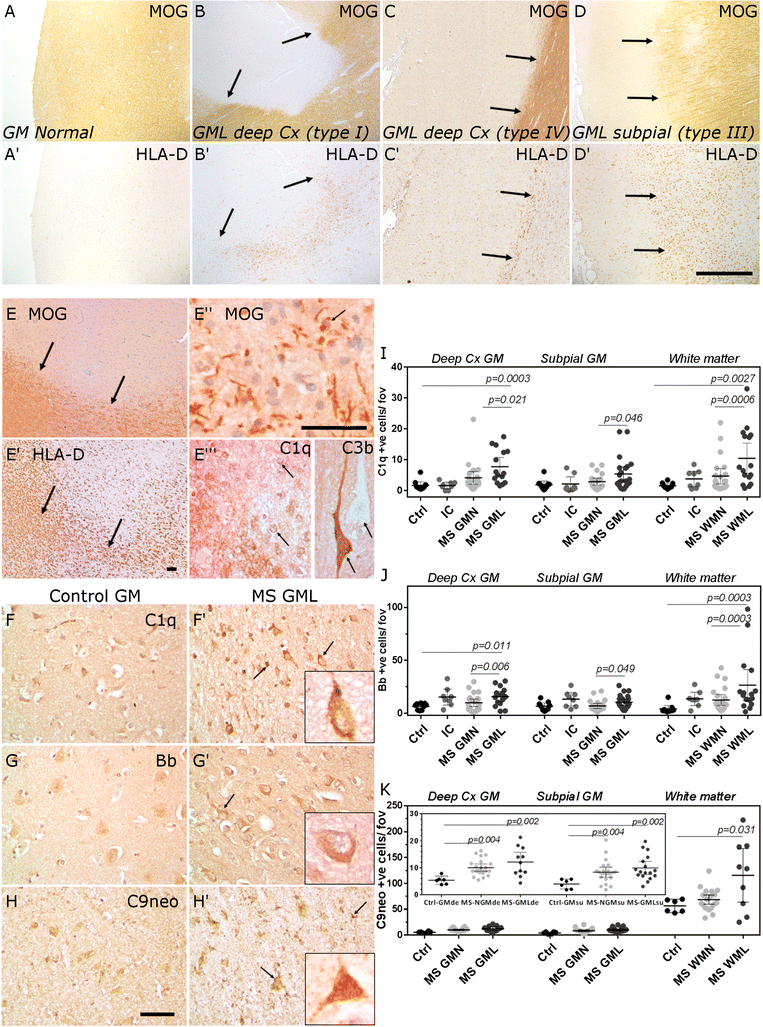
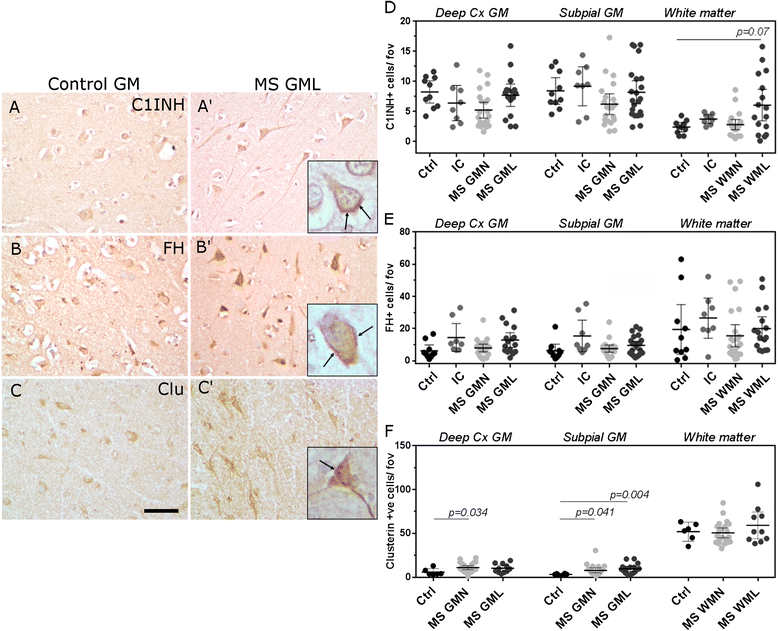
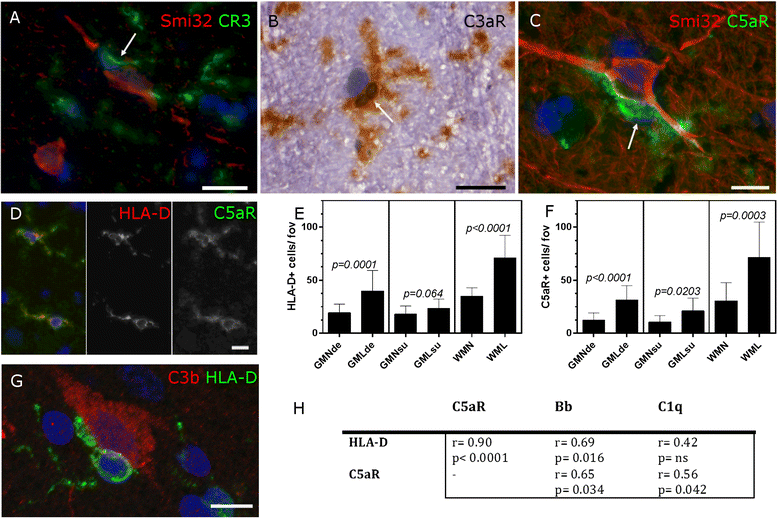
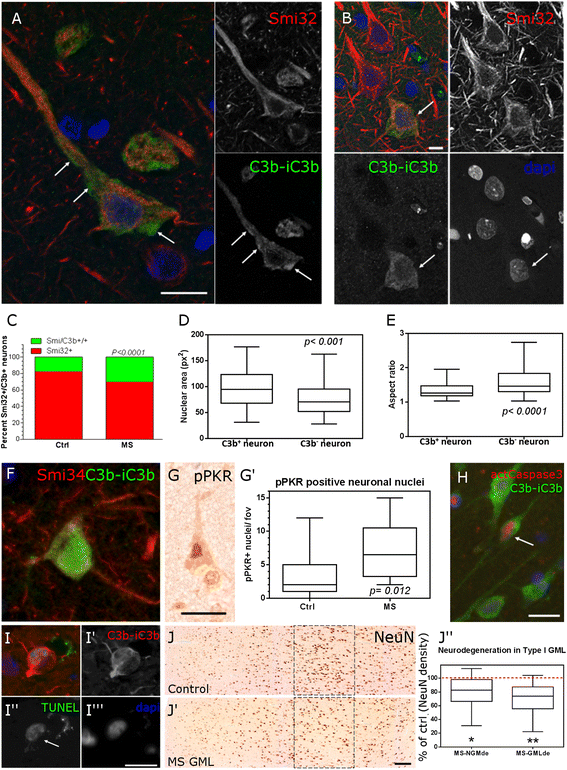
Results: Expression of the transcript for C1qA was noted in neurons and the activation fragment and opsonin C3b-labelled neurons and glia in the MS cortical and deep grey matter. The density of immunostained cells positive for the classical complement pathway protein C1q and the alternative complement pathway activation fragment Bb was significantly increased in cortical grey matter lesions in comparison to control grey matter. The number of cells immunostained for the membrane attack complex was elevated in cortical lesions, indicating complement activation to completion. The numbers of classical (C1-inhibitor) and alternative (factor H) pathway regulator-positive cells were unchanged between MS and controls, whilst complement anaphylatoxin receptor-bearing microglia in the MS cortex were found closely apposed to cortical neurons. Complement immunopositive neurons displayed an altered nuclear morphology, indicative of cell stress/damage, supporting our finding of significant neurodegeneration in cortical grey matter lesions.
Conclusions: Complement is activated in the MS cortical grey matter lesions in areas of elevated numbers of complement receptor-positive microglia and suggests that complement over-activation may contribute to the worsening pathology that underlies the irreversible progression of MS.
Keywords: Complement; Grey matter lesion; Innate immunity; Multiple sclerosis; Neurodegeneration.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

