Epigenetic mechanisms in neurogenesis
- PMID: 27334043
- PMCID: PMC5610421
- DOI: 10.1038/nrn.2016.70
Epigenetic mechanisms in neurogenesis
Abstract
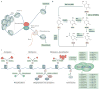
In the embryonic and adult brain, neural stem cells proliferate and give rise to neurons and glia through highly regulated processes. Epigenetic mechanisms - including DNA and histone modifications, as well as regulation by non-coding RNAs - have pivotal roles in different stages of neurogenesis. Aberrant epigenetic regulation also contributes to the pathogenesis of various brain disorders. Here, we review recent advances in our understanding of epigenetic regulation in neurogenesis and its dysregulation in brain disorders, including discussion of newly identified DNA cytosine modifications. We also briefly cover the emerging field of epitranscriptomics, which involves modifications of mRNAs and long non-coding RNAs.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Waddington CH. An Introduction to Modern Genetics. G. Allen & Unwin ltd; 1939. This is the first time that the term ‘epigenetics’ was proposed.
-
- Fu Y, Dominissini D, Rechavi G, He C. Gene expression regulation mediated through reversible m6A RNA methylation. Nat Rev Genet. 2014;15:293–306. - PubMed
-
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. - PubMed
Publication types
MeSH terms
Grants and funding
- RF1 AG052476/AG/NIA NIH HHS/United States
- R01 NS079625/NS/NINDS NIH HHS/United States
- R37 NS047344/NS/NINDS NIH HHS/United States
- R21 NS095348/NS/NINDS NIH HHS/United States
- R21 MH110160/MH/NIMH NIH HHS/United States
- R56 NS047344/NS/NINDS NIH HHS/United States
- R01 NS048271/NS/NINDS NIH HHS/United States
- R01 NS047344/NS/NINDS NIH HHS/United States
- R01 NS051630/NS/NINDS NIH HHS/United States
- R21 HD086820/HD/NICHD NIH HHS/United States
- R01 MH102690/MH/NIMH NIH HHS/United States
- R01 MH105128/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

