Cytochrome P450 metabolism of the post-lanosterol intermediates explains enigmas of cholesterol synthesis
- PMID: 27334049
- PMCID: PMC4917857
- DOI: 10.1038/srep28462
Cytochrome P450 metabolism of the post-lanosterol intermediates explains enigmas of cholesterol synthesis
Abstract
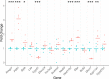
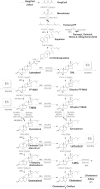
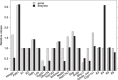
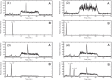
Cholesterol synthesis is among the oldest metabolic pathways, consisting of the Bloch and Kandutch-Russell branches. Following lanosterol, sterols of both branches are proposed to be dedicated to cholesterol. We challenge this dogma by mathematical modeling and with experimental evidence. It was not possible to explain the sterol profile of testis in cAMP responsive element modulator tau (Crem τ) knockout mice with mathematical models based on textbook pathways of cholesterol synthesis. Our model differs in the inclusion of virtual sterol metabolizing enzymes branching from the pathway. We tested the hypothesis that enzymes from the cytochrome P450 (CYP) superfamily can participate in the catalysis of non-classical reactions. We show that CYP enzymes can metabolize multiple sterols in vitro, establishing novel branching points of cholesterol synthesis. In conclusion, sterols of cholesterol synthesis can be oxidized further to metabolites not dedicated to production of cholesterol. Additionally, CYP7A1, CYP11A1, CYP27A1, and CYP46A1 are parts of a broader cholesterol synthesis network.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

