Vitamin D and omega-3 fatty acid supplements in children with autism spectrum disorder: a study protocol for a factorial randomised, double-blind, placebo-controlled trial
- PMID: 27334138
- PMCID: PMC4917935
- DOI: 10.1186/s13063-016-1428-8
Vitamin D and omega-3 fatty acid supplements in children with autism spectrum disorder: a study protocol for a factorial randomised, double-blind, placebo-controlled trial
Abstract
Background: There is strong mechanistic evidence to suggest that vitamin D and omega-3 long chain polyunsaturated fatty acids (n-3 LCPUFAs), specifically docosahexaenoic acid (DHA), have the potential to significantly improve the symptoms of autism spectrum disorder (ASD). However, there are no trials that have measured the effect of both vitamin D and n-3 LCPUFA supplementation on autism severity symptoms. The objective of this 2 × 2 factorial trial is to investigate the effect of vitamin D, n-3 LCPUFAs or a combination of both on core symptoms of ASD.
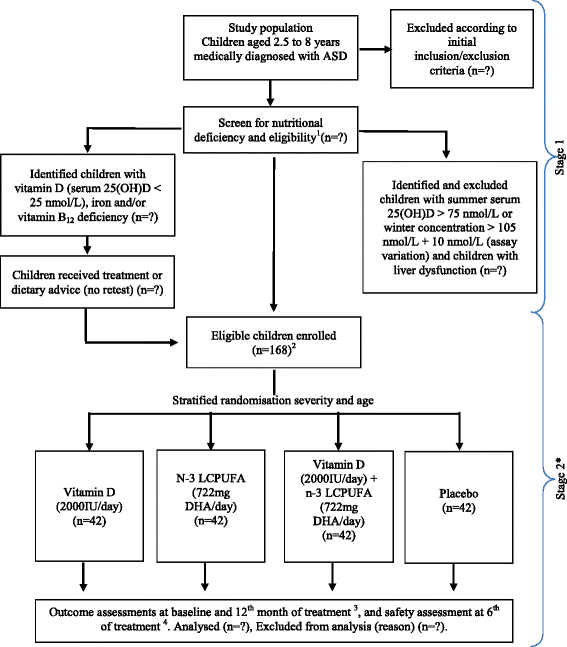
Methods/design: Children with ASD living in New Zealand (n = 168 children) will be randomised to one of four treatments daily: vitamin D (2000 IU), n-3 LCPUFAs (722 mg DHA), vitamin D (2000 IU) + n-3 LCPUFAs (722 mg DHA) or placebo for 12 months. All researchers, participants and their caregivers will be blinded until the data analysis is completed, and randomisation of the active/placebo capsules and allocation will be fully concealed from all mentioned parties. The primary outcome measures are the change in social-communicative functioning, sensory processing issues and problem behaviours between baseline and 12 months. A secondary outcome measure is the effect on gastrointestinal symptoms. Baseline data will be used to assess and correct basic nutritional deficiencies prior to treatment allocation. For safety measures, serum 25-hydroxyvitamin D 25(OH)D and calcium will be monitored at baseline, 6 and 12 months, and weekly compliance and gastrointestinal symptom diaries will be completed by caregivers throughout the study period.
Discussion: To our knowledge there are no randomised controlled trials assessing the effects of both vitamin D and DHA supplementation on core symptoms of ASD. If it is shown that either vitamin D, DHA or both are effective, the trial would reveal a non-invasive approach to managing ASD symptoms.
Trial registration: Australian New Zealand Clinical Trial Registry, ACTRN12615000144516 . Registered on 16 February 2015.
Keywords: ASD; Autism; Omega-3 fatty acids; Supplements; Vitamin D.
Figures
References
-
- New Zealand Guidelines Group . What does ASD look like? A resource to help identify autism spectrum disorder. Wellington: New Zealand Guidelines Group; 2010.
-
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5™ (5th ed). Washinton, DC; 2013
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

