Hydration Repulsion between Carbohydrate Surfaces Mediated by Temperature and Specific Ions
- PMID: 27334145
- PMCID: PMC4917866
- DOI: 10.1038/srep28553
Hydration Repulsion between Carbohydrate Surfaces Mediated by Temperature and Specific Ions
Abstract
Stabilizing colloids or nanoparticles in solution involves a fine balance between surface charges, steric repulsion of coating molecules, and hydration forces against van der Waals attractions. At high temperature and electrolyte concentrations, the colloidal stability of suspensions usually decreases rapidly. Here, we report a new experimental and simulation discovery that the polysaccharide (dextran) coated nanoparticles show ion-specific colloidal stability at high temperature, where we observed enhanced colloidal stability of nanoparticles in CaCl2 solution but rapid nanoparticle-nanoparticle aggregation in MgCl2 solution. The microscopic mechanism was unveiled in atomistic simulations. The presence of surface bound Ca(2+) ions increases the carbohydrate hydration and induces strongly polarized repulsive water structures beyond at least three hydration shells which is farther-reaching than previously assumed. We believe leveraging the binding of strongly hydrated ions to macromolecular surfaces represents a new paradigm in achieving absolute hydration and colloidal stability for a variety of materials, particularly under extreme conditions.
Figures


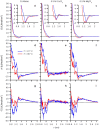
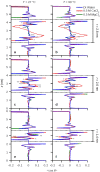

References
-
- Israelachvili J. & Wennerstrom H. Role of hydration and water structure in biological and colloidal interactions. Nature 379, 219–225 (1996). - PubMed
-
- Otting G., Liepinsh E. & Wuthrich K. Protein hydration in aqueous solution. Science 254, 974–980 (1991). - PubMed
-
- Zaks A. & Klibanov A. M. The effect of water on enzyme action in organic media. J. Biol. Chem. 263, 8017–8021 (1988). - PubMed
-
- Raviv U. & Klein J. Fluidity of bound hydration layers. Science 297, 1540–1543 (2002). - PubMed
-
- LeNeveu D. & Rand R. P. Measurement of forces between lecithin bilayers. Nature 259, 601–603 (1976). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

