Apoptosis-Resistant Cardiac Progenitor Cells Modified With Apurinic/Apyrimidinic Endonuclease/Redox Factor 1 Gene Overexpression Regulate Cardiac Repair After Myocardial Infarction
- PMID: 27334489
- PMCID: PMC4954451
- DOI: 10.5966/sctm.2015-0281
Apoptosis-Resistant Cardiac Progenitor Cells Modified With Apurinic/Apyrimidinic Endonuclease/Redox Factor 1 Gene Overexpression Regulate Cardiac Repair After Myocardial Infarction
Abstract
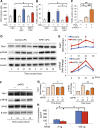
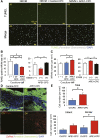
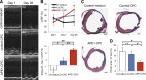
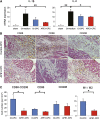
: Overcoming the insufficient survival of cell grafts is an essential objective in cell-based therapy. Apurinic/apyrimidinic endonuclease/redox factor 1 (APE1) promotes cell survival and may enhance the therapeutic effect of engrafted cells. The aim of this study is to determine whether APE1 overexpression in cardiac progenitor cells (CPCs) could ameliorate the efficiency of cell-based therapy. CPCs isolated from 8- to 10-week-old C57BL/6 mouse hearts were infected with retrovirus harboring APE1-DsRed (APE1-CPC) or a DsRed control (control-CPC). Oxidative stress-induced apoptosis was then assessed in APE1-CPCs, control-CPCs, and neonatal rat ventricular myocytes (NRVMs) cocultured with these CPCs. This analysis revealed that APE1 overexpression inhibited CPC apoptosis with activation of transforming growth factor β-activated kinase 1 (TAK1) and nuclear factor (NF)-κB. In the coculture model, NRVM apoptosis was inhibited to a greater extent in the presence of APE1-CPCs compared with control-CPCs. Moreover, the number of surviving DsRed-positive CPC grafts was significantly higher 7 days after the transplant of APE1-CPCs into a mouse myocardial infarction model, and the left ventricular ejection fraction showed greater improvement with attenuation of fibrosis 28 days after the transplant of APE1-CPCs compared with control-CPCs. Additionally, fewer inflammatory macrophages and a higher percentage of cardiac α-sarcomeric actinin-positive CPC-grafts were observed in mice injected with APE1-CPCs compared with control-CPCs after 7 days. In conclusion, antiapoptotic APE1-CPC graft, which increased TAK1-NF-κB pathway activation, survived effectively in the ischemic heart, restored cardiac function, and reduced cardiac inflammation and fibrosis. APE1 overexpression in CPCs may serve as a novel strategy to improve cardiac cell therapy.
Significance: Improving the survival of cell grafts is essential to maximize the efficacy of cell therapy. The authors investigated the role of APE1 in CPCs under ischemic conditions and evaluated the therapeutic efficacy of transplanted APE1-overexpressing CPCs in a mouse model of myocardial infarction. APE1 hindered apoptosis in CPC grafts subjected to oxidative stress caused in part by increased TAK1-NF-κB pathway activation. Furthermore, APE1-CPC grafts that effectively survived in the ischemic heart restored cardiac function and attenuated fibrosis through pleiotropic mechanisms that remain to be characterized. These findings suggest that APE1 overexpression in CPCs may be a novel strategy to reinforce cardiac cell therapy.
Keywords: Apoptosis; Cellular therapy; Gene expression; Sca-1; Stem/progenitor cell.
©AlphaMed Press.
Figures

References
-
- Assmus B, Honold J, Schächinger V, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. - PubMed
-
- Stamm C, Kleine HD, Choi YH, et al. Intramyocardial delivery of CD133+ bone marrow cells and coronary artery bypass grafting for chronic ischemic heart disease: Safety and efficacy studies. J Thorac Cardiovasc Surg. 2007;133:717–725. - PubMed
-
- Menasché P, Alfieri O, Janssens S, et al. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: First randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189–1200. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

