Generation of a Bone Organ by Human Adipose-Derived Stromal Cells Through Endochondral Ossification
- PMID: 27334490
- PMCID: PMC4954448
- DOI: 10.5966/sctm.2015-0256
Generation of a Bone Organ by Human Adipose-Derived Stromal Cells Through Endochondral Ossification
Abstract
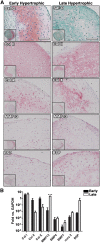
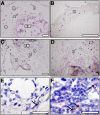
: Recapitulation of endochondral ossification (ECO) (i.e., generation of marrow-containing ossicles through a cartilage intermediate) has relevance to develop human organotypic models for bone or hematopoietic cells and to engineer grafts for bone regeneration. Unlike bone marrow-derived stromal cells (also known as bone marrow-derived mesenchymal stromal/stem cells), adipose-derived stromal cells (ASC) have so far failed to form a bone organ by ECO. The goal of the present study was to assess whether priming human ASC to a defined stage of chondrogenesis in vitro allows their autonomous ECO upon ectopic implantation. ASC were cultured either as micromass pellets or into collagen sponges in chondrogenic medium containing transforming growth factor-β3 and bone morphogenetic protein-6 for 4 weeks (early hypertrophic templates) or for two additional weeks in medium supplemented with β-glycerophosphate, l-thyroxin, and interleukin1-β to induce hypertrophic maturation (late hypertrophic templates). Constructs were implanted in vivo and analyzed after 8 weeks. In vitro, ASC deposited cartilaginous matrix positive for glycosaminoglycans, type II collagen, and Indian hedgehog. Hypertrophic maturation induced upregulation of type X collagen, bone sialoprotein, and matrix metalloproteinase13 (MMP13). In vivo, both early and late hypertrophic templates underwent cartilage remodeling, as assessed by MMP13- and tartrate-resistant acid phosphatase-positive staining, and developed bone ossicles, including bone marrow elements, although to variable degrees of efficiency. In situ hybridization for human-specific sequences and staining with a human specific anti-CD146 antibody demonstrated the direct contribution of ASC to bone and stromal tissue formation. In conclusion, despite their debated skeletal progenitor nature, human ASC can generate bone organs through ECO when suitably primed in vitro.
Significance: Recapitulation of endochondral ossification (ECO) (i.e., generation of marrow-containing ossicles through a cartilage intermediate) has relevance to develop human organotypic models for bone or hematopoietic cells and to engineer grafts for bone regeneration. This study demonstrated that expanded, human adult adipose-derived stromal cells can generate ectopic bone through ECO, as previously reported for bone marrow stromal cells. This system can be used as a model in a variety of settings for mimicking ECO during development, physiology, or pathology (e.g., to investigate the role of BMPs, their receptors, and signaling pathways). The findings have also translational relevance in the field of bone regeneration, which, despite several advances in the domains of materials and surgical techniques, still faces various limitations before being introduced in the routine clinical practice.
Keywords: Adipose-derived stromal cells; Bone organ; Differentiation; Endochondral ossification; Tissue engineering.
©AlphaMed Press.
Figures




References
-
- Shapiro F. Bone development and its relation to fracture repair. The role of mesenchymal osteoblasts and surface osteoblasts. Eur Cell Mater. 2008;15:53–76. - PubMed
-
- Giannoudis PV, Kanakaris NK, Tsiridis E. Principles of internal fixation and selection of implants for periprosthetic femoral fractures. Injury. 2007;38:669–687. - PubMed
-
- Papadimitropoulos A, Scotti C, Bourgine P, et al. Engineered decellularized matrices to instruct bone regeneration processes. Bone. 2015;70:66–72. - PubMed
-
- Rosset P, Deschaseaux F, Layrolle P. Cell therapy for bone repair. Orthop Traumatol Surg Res. 2014;100(suppl):S107–S112. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

