Efficacy and safety profile of linezolid in the treatment of multidrug-resistant (MDR) and extensively drug-resistant (XDR) tuberculosis: a systematic review and meta-analysis
- PMID: 27334498
- PMCID: PMC4917997
- DOI: 10.1186/s12941-016-0156-y
Efficacy and safety profile of linezolid in the treatment of multidrug-resistant (MDR) and extensively drug-resistant (XDR) tuberculosis: a systematic review and meta-analysis
Abstract
Background: Treatment options for drug-resistant tuberculosis are still limited. Linezolid has been recommended for treatment of patients with multidrug-resistant (MDR) or extensively-drug-resistant (XDR) tuberculosis, although uncertainties remain regarding its safety and tolerability in these circumstances.
Objective: To systematically evaluate the existing evidence regarding the efficacy and tolerability of linezolid in the treatment of MDR or XDR tuberculosis.
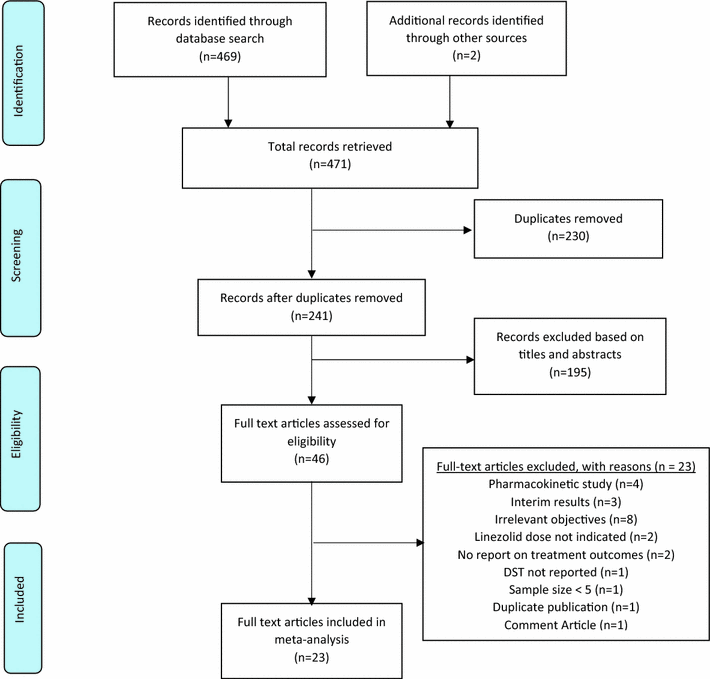
Methods: We conducted a systematic review and meta-analysis in accordance with the PRISMA guidelines. Searches were conducted in PubMed, Web of Science and EMBASE followed by direct search of abstracts in the International Journal of Tuberculosis and Lung Disease to retrieve primary studies published between January 2000 and January 2016 assessing linezolid efficacy and safety in the treatment of drug-resistant TB. We evaluated the occurrence of outcomes including culture conversion, treatment success and incidence of adverse events such as myelosuppression and neuropathy.
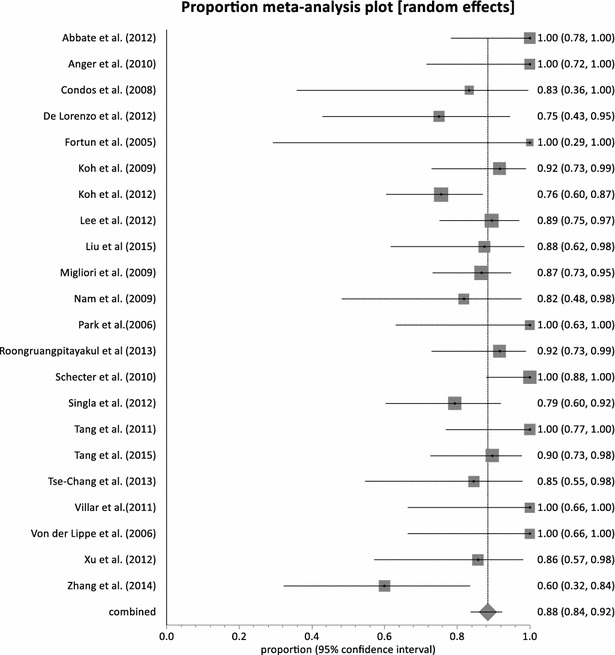
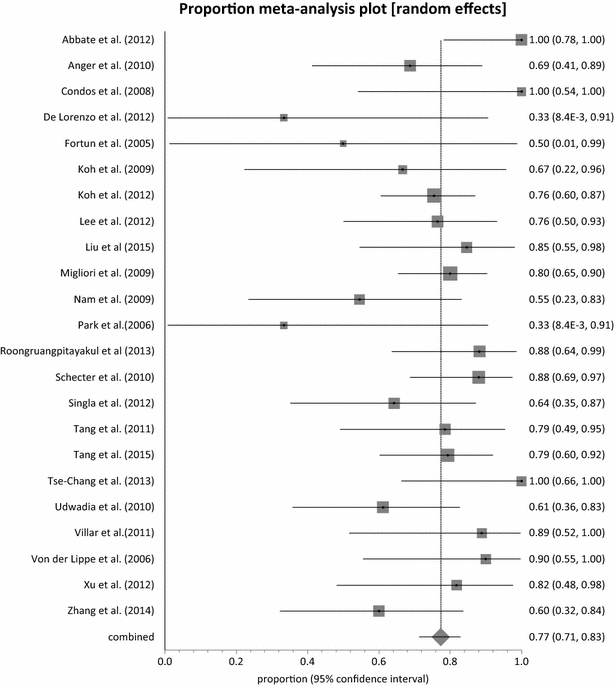
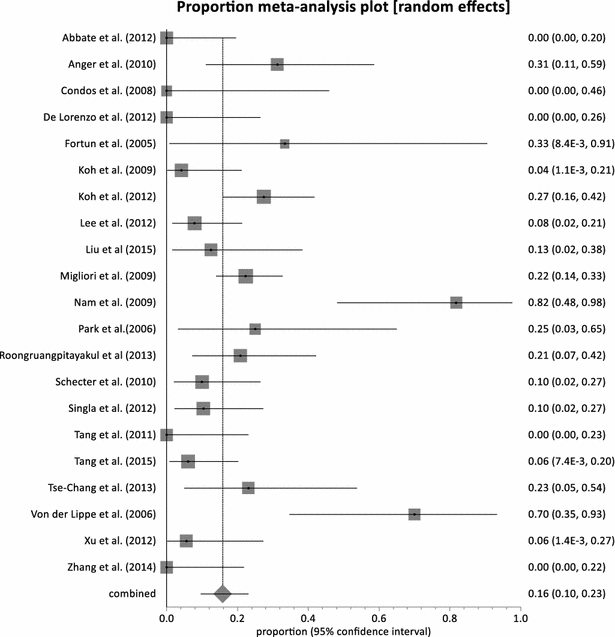
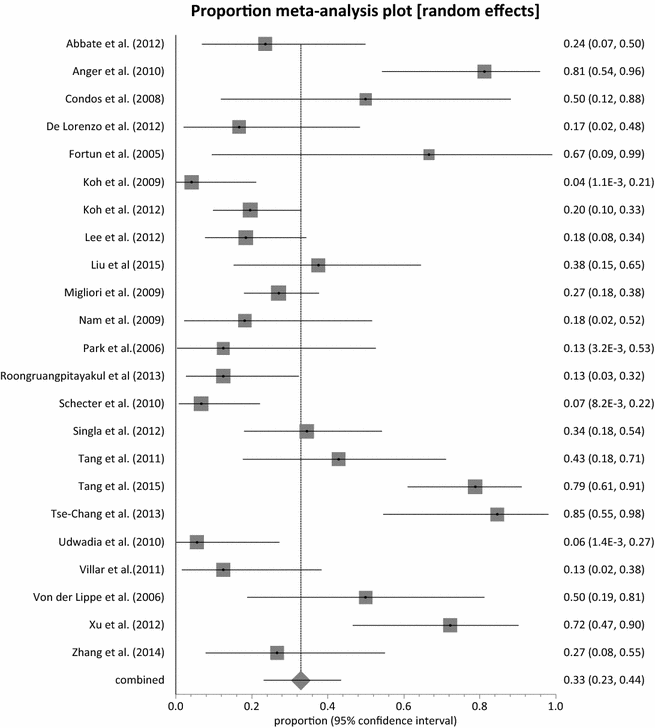
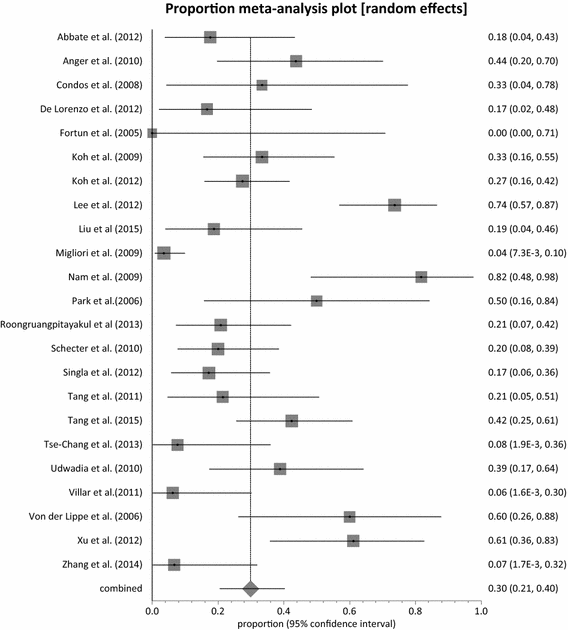
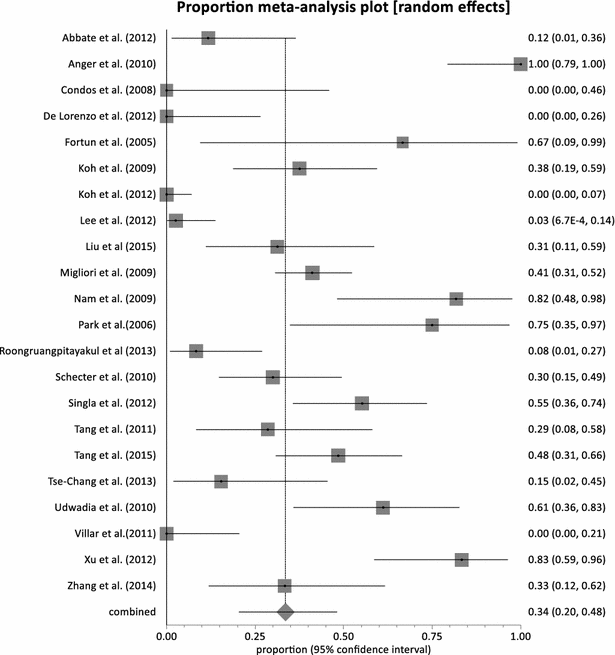
Results: Twenty-three (23) studies conducted in fourteen (14) countries and involving 507 patients were retrieved. Only 1 randomized controlled trial was identified and none of the identified studies involved participants from Africa. The pooled proportion for treatment success was 77.36 % (95 % CI = 71.38-82.83 %, I(2) = 37.6 %) with culture conversion rate determined as 88.45 % (95 % CI = 83.82-92.38 %, I(2) = 45.4 %). There was no strong evidence for both culture conversion (p = 0.0948) and treatment success (p = 0.0695) between linezolid daily doses ≤ 600 and > 600 mg. Only myelosuppression showed a strong statistical significance (p < 0.0001) between dose comparisons. The incidence of neuropathy and other adverse events leading to permanent discontinuation of linezolid also showed no significance upon dose comparisons (p = 0.3213, p = 0.9050 respectively).
Conclusion: Available evidence presents Linezolid as a viable option in the treatment of MDR/XDR TB although patients ought to be monitored closely for the incidence of major adverse events such as myelosuppression and neuropathy. Additionally, highly powered randomized controlled trials including participants from endemic regions are urgently needed to better inform the magnitude and significance of Linezolid treatment effect in MDR and XDR TB patients.
Keywords: Drug therapy; Extensively drug resistant; Infectious diseases; Linezolid; Meta-analysis; Multi-drug resistance; Tuberculosis.
Figures
Similar articles
-
Linezolid for the treatment of complicated drug-resistant tuberculosis: a systematic review and meta-analysis.Int J Tuberc Lung Dis. 2012 Apr;16(4):447-54. doi: 10.5588/ijtld.11.0451. Int J Tuberc Lung Dis. 2012. PMID: 22325685
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Xpert MTB/XDR for detection of pulmonary tuberculosis and resistance to isoniazid, fluoroquinolones, ethionamide, and amikacin.Cochrane Database Syst Rev. 2022 May 18;5(5):CD014841. doi: 10.1002/14651858.CD014841.pub2. Cochrane Database Syst Rev. 2022. PMID: 35583175 Free PMC article.
Cited by
-
Therapeutic Drug Monitoring of Linezolid in Drug-Resistant Tuberculosis Patients: Clinical Factors and Hematological Toxicities.Infect Drug Resist. 2024 Jun 20;17:2531-2540. doi: 10.2147/IDR.S464429. eCollection 2024. Infect Drug Resist. 2024. PMID: 38933777 Free PMC article.
-
Linezolid-induced hematemesis, a rare and life-threatening adverse reaction. A case report of Karonga district in Malawi.Oxf Med Case Reports. 2023 Mar 25;2023(3):omad020. doi: 10.1093/omcr/omad020. eCollection 2023 Mar. Oxf Med Case Reports. 2023. PMID: 36993836 Free PMC article. No abstract available.
-
Drug-associated adverse events in the treatment of multidrug-resistant tuberculosis: an individual patient data meta-analysis.Lancet Respir Med. 2020 Apr;8(4):383-394. doi: 10.1016/S2213-2600(20)30047-3. Epub 2020 Mar 17. Lancet Respir Med. 2020. PMID: 32192585 Free PMC article.
-
Update on Toxic Neuropathies.Curr Treat Options Neurol. 2022 May;24(5):203-216. doi: 10.1007/s11940-022-00716-5. Epub 2022 Apr 6. Curr Treat Options Neurol. 2022. PMID: 36186669 Free PMC article.
-
Revisiting the β-Lactams for Tuberculosis Therapy with a Compound-Compound Synthetic Lethality Approach.Antimicrob Agents Chemother. 2019 Oct 22;63(11):e01319-19. doi: 10.1128/AAC.01319-19. Print 2019 Nov. Antimicrob Agents Chemother. 2019. PMID: 31427291 Free PMC article.
References
-
- WHO. Global tuberculosis report 2015. http://www.apps.who.int/iris/bitstream/10665/191102/1/9789241565059_eng.... Accessed 2 Feb 2016.
-
- WHO. Tuberculosis. http://www.who.int/mediacentre/factsheets/fs104/en/ Accessed 2 Feb 2016.
-
- WHO. Tuberculosis: the global burden 2005. http://www.who.int/tb/publications/tb_global_facts_sep05_en.pdf Accessed 2 Feb 2016.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

