DC-SIGN and L-SIGN Are Attachment Factors That Promote Infection of Target Cells by Human Metapneumovirus in the Presence or Absence of Cellular Glycosaminoglycans
- PMID: 27334579
- PMCID: PMC4988148
- DOI: 10.1128/JVI.00537-16
DC-SIGN and L-SIGN Are Attachment Factors That Promote Infection of Target Cells by Human Metapneumovirus in the Presence or Absence of Cellular Glycosaminoglycans
Abstract
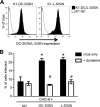
It is well established that glycosaminoglycans (GAGs) function as attachment factors for human metapneumovirus (HMPV), concentrating virions at the cell surface to promote interaction with other receptors for virus entry and infection. There is increasing evidence to suggest that multiple receptors may exhibit the capacity to promote infectious entry of HMPV into host cells; however, definitive identification of specific transmembrane receptors for HMPV attachment and entry is complicated by the widespread expression of cell surface GAGs. pgsA745 Chinese hamster ovary (CHO) cells are deficient in the expression of cell surface GAGs and resistant to HMPV infection. Here, we demonstrate that the expression of the Ca(2+)-dependent C-type lectin receptor (CLR) DC-SIGN (CD209L) or L-SIGN (CD209L) rendered pgsA745 cells permissive to HMPV infection. Unlike infection of parental CHO cells, HMPV infection of pgsA745 cells expressing DC-SIGN or L-SIGN was dynamin dependent and inhibited by mannan but not by pretreatment with bacterial heparinase. Parental CHO cells expressing DC-SIGN/L-SIGN also showed enhanced susceptibility to dynamin-dependent HMPV infection, confirming that CLRs can promote HMPV infection in the presence or absence of GAGs. Comparison of pgsA745 cells expressing wild-type and endocytosis-defective mutants of DC-SIGN/L-SIGN indicated that the endocytic function of CLRs was not essential but could contribute to HMPV infection of GAG-deficient cells. Together, these studies confirm a role for CLRs as attachment factors and entry receptors for HMPV infection. Moreover, they define an experimental system that can be exploited to identify transmembrane receptors and entry pathways where permissivity to HMPV infection can be rescued following the expression of a single cell surface receptor.
Importance: On the surface of CHO cells, glycosaminoglycans (GAGs) function as the major attachment factor for human metapneumoviruses (HMPV), promoting dynamin-independent infection. Consistent with this, GAG-deficient pgaA745 CHO cells are resistant to HMPV. However, expression of DC-SIGN or L-SIGN rendered pgsA745 cells permissive to dynamin-dependent infection by HMPV, although the endocytic function of DC-SIGN/L-SIGN was not essential for, but could contribute to, enhanced infection. These studies provide direct evidence implicating DC-SIGN/L-SIGN as an alternate attachment factor for HMPV attachment, promoting dynamin-dependent infection via other unknown receptors in the absence of GAGs. Moreover, we describe a unique experimental system for the assessment of putative attachment and entry receptors for HMPV.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures





Similar articles
-
Endocytic function is critical for influenza A virus infection via DC-SIGN and L-SIGN.Sci Rep. 2016 Jan 14;6:19428. doi: 10.1038/srep19428. Sci Rep. 2016. PMID: 26763587 Free PMC article.
-
N-linked glycosylation facilitates sialic acid-independent attachment and entry of influenza A viruses into cells expressing DC-SIGN or L-SIGN.J Virol. 2011 Mar;85(6):2990-3000. doi: 10.1128/JVI.01705-10. Epub 2010 Dec 29. J Virol. 2011. PMID: 21191006 Free PMC article.
-
The C-type Lectin Langerin Functions as a Receptor for Attachment and Infectious Entry of Influenza A Virus.J Virol. 2015 Oct 14;90(1):206-21. doi: 10.1128/JVI.01447-15. Print 2016 Jan 1. J Virol. 2015. PMID: 26468543 Free PMC article.
-
DC-SIGN: binding receptor for HCV?World J Gastroenterol. 2004 Apr 1;10(7):925-9. doi: 10.3748/wjg.v10.i7.925. World J Gastroenterol. 2004. PMID: 15052667 Free PMC article. Review.
-
The C type lectins DC-SIGN and L-SIGN: receptors for viral glycoproteins.Methods Mol Biol. 2007;379:51-68. doi: 10.1007/978-1-59745-393-6_4. Methods Mol Biol. 2007. PMID: 17502670 Free PMC article. Review.
Cited by
-
Low-density lipoprotein receptor-related protein 1 (LRP1) as an auxiliary host factor for RNA viruses.Life Sci Alliance. 2023 Apr 18;6(7):e202302005. doi: 10.26508/lsa.202302005. Print 2023 Jul. Life Sci Alliance. 2023. PMID: 37072184 Free PMC article.
-
Receptors and host factors: key players in human metapneumovirus infection.Front Cell Infect Microbiol. 2025 Apr 1;15:1557880. doi: 10.3389/fcimb.2025.1557880. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40235933 Free PMC article. Review.
-
Targeting Crucial Host Factors of SARS-CoV-2.ACS Infect Dis. 2020 Nov 13;6(11):2844-2865. doi: 10.1021/acsinfecdis.0c00456. Epub 2020 Oct 28. ACS Infect Dis. 2020. PMID: 33112126 Free PMC article. Review.
-
Meeting report of the 37th International Conference on Antiviral Research in Gold Coast, Australia, May 20-24, 2024, organized by the International Society for Antiviral Research.Antiviral Res. 2024 Dec;232:106037. doi: 10.1016/j.antiviral.2024.106037. Epub 2024 Nov 13. Antiviral Res. 2024. PMID: 39542140
-
MARCH8 Restricts Influenza A Virus Infectivity but Does Not Downregulate Viral Glycoprotein Expression at the Surface of Infected Cells.mBio. 2021 Oct 26;12(5):e0148421. doi: 10.1128/mBio.01484-21. Epub 2021 Sep 14. mBio. 2021. PMID: 34517760 Free PMC article.
References
-
- Kuiken T, van den Hoogen BG, van Riel DA, Laman JD, van Amerongen G, Sprong L, Fouchier RA, Osterhaus AD. 2004. Experimental human metapneumovirus infection of cynomolgus macaques (Macaca fascicularis) results in virus replication in ciliated epithelial cells and pneumocytes with associated lesions throughout the respiratory tract. Am J Pathol 164:1893–1900. doi:10.1016/S0002-9440(10)63750-9. - DOI - PMC - PubMed
-
- Kolli D, Gupta MR, Sbrana E, Velayutham TS, Chao H, Casola A, Garofalo RP. 2014. Alveolar macrophages contribute to the pathogenesis of human metapneumovirus infection while protecting against respiratory syncytial virus infection. Am J Respir Cell Mol Biol 51:502–515. doi:10.1165/rcmb.2013-0414OC. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

