Adeno-associated Virus Vectors Efficiently Transduce Mouse and Rabbit Sensory Neurons Coinfected with Herpes Simplex Virus 1 following Peripheral Inoculation
- PMID: 27334582
- PMCID: PMC4988153
- DOI: 10.1128/JVI.01028-16
Adeno-associated Virus Vectors Efficiently Transduce Mouse and Rabbit Sensory Neurons Coinfected with Herpes Simplex Virus 1 following Peripheral Inoculation
Abstract
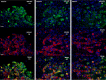
Following infection of epithelial tissues, herpes simplex virus 1 (HSV-1) virions travel via axonal transport to sensory ganglia and establish a lifelong latent infection within neurons. Recent studies have revealed that, following intraganglionic or intrathecal injection, recombinant adeno-associated virus (rAAV) vectors can also infect sensory neurons and are capable of stable, long-term transgene expression. We sought to determine if application of rAAV to peripheral nerve termini at the epithelial surface would allow rAAV to traffic to sensory ganglia in a manner similar to that seen with HSV. We hypothesized that footpad or ocular inoculation with rAAV8 would result in transduction of dorsal root ganglia (DRG) or trigeminal ganglia (TG), respectively. To test this, we inoculated the footpads of mice with various amounts of rAAV as well as rAAV capsid mutants. We demonstrated that this method of inoculation can achieve a transduction rate of >90% of the sensory neurons in the DRG that innervate the footpad. Similarly, we showed that corneal inoculation with rAAV vectors in the rabbit efficiently transduced >70% of the TG neurons in the optic tract. Finally, we demonstrated that coinfection of mouse footpads or rabbit eyes with rAAV vectors and HSV-1 resulted in colocalization in nearly all of the HSV-1-positive neurons. These results suggest that rAAV is a useful tool for the study of HSV-1 infection and may provide a means to deliver therapeutic cargos for the treatment of HSV infections or of dysfunctions of sensory ganglia.
Importance: Adeno-associated virus (AAV) has been shown to transduce dorsal root ganglion sensory neurons following direct intraganglionic sciatic nerve injection and intraperitoneal and intravenous injection as well as intrathecal injection. We sought to determine if rAAV vectors would be delivered to the same sensory neurons that herpes simplex virus (HSV-1) infects when applied peripherally at an epithelial surface that had been treated to expose the underlying sensory nerve termini. For this study, we chose two well-established HSV-1 infection models: mouse footpad infection and rabbit ocular infection. The results presented here provide the first description of AAV vectors transducing neurons following delivery at the skin/epithelium/eye. The ability of AAV to cotransduce HSV-1-infected neurons in both the mouse and the rabbit provides an opportunity to experimentally explore and disrupt host and viral proteins that are integral to the establishment of HSV-1 latency, to the maintenance of latency, and to reactivation from latency in vivo.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures





References
-
- Margolis TP, Dawson CR, LaVail JH. 1992. Herpes simplex viral infection of the mouse trigeminal ganglion. Immunohistochemical analysis of cell populations. Invest Ophthalmol Vis Sci 33:259–267. - PubMed
-
- Lovric J, Mano M, Zentilin L, Eulalio A, Zacchigna S, Giacca M. 2012. Terminal differentiation of cardiac and skeletal myocytes induces permissivity to AAV transduction by relieving inhibition imposed by DNA damage response proteins. Mol Ther 20:2087–2097. doi: 10.1038/mt.2012.144. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

