Phosphorylation of the Brome Mosaic Virus Capsid Regulates the Timing of Viral Infection
- PMID: 27334588
- PMCID: PMC4988143
- DOI: 10.1128/JVI.00833-16
Phosphorylation of the Brome Mosaic Virus Capsid Regulates the Timing of Viral Infection
Abstract
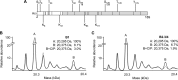
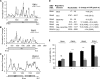
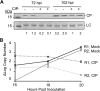
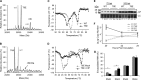
The four brome mosaic virus (BMV) RNAs (RNA1 to RNA4) are encapsidated in three distinct virions that have different disassembly rates in infection. The mechanism for the differential release of BMV RNAs from virions is unknown, since 180 copies of the same coat protein (CP) encapsidate each of the BMV genomic RNAs. Using mass spectrometry, we found that the BMV CP contains a complex pattern of posttranslational modifications. Treatment with phosphatase was found to not significantly affect the stability of the virions containing RNA1 but significantly impacted the stability of the virions that encapsidated BMV RNA2 and RNA3/4. Cryo-electron microscopy reconstruction revealed dramatic structural changes in the capsid and the encapsidated RNA. A phosphomimetic mutation in the flexible N-terminal arm of the CP increased BMV RNA replication and virion production. The degree of phosphorylation modulated the interaction of CP with the encapsidated RNA and the release of three of the BMV RNAs. UV cross-linking and immunoprecipitation methods coupled to high-throughput sequencing experiments showed that phosphorylation of the BMV CP can impact binding to RNAs in the virions, including sequences that contain regulatory motifs for BMV RNA gene expression and replication. Phosphatase-treated virions affected the timing of CP expression and viral RNA replication in plants. The degree of phosphorylation decreased when the plant hosts were grown at an elevated temperature. These results show that phosphorylation of the capsid modulates BMV infection.
Importance: How icosahedral viruses regulate the release of viral RNA into the host is not well understood. The selective release of viral RNA can regulate the timing of replication and gene expression. Brome mosaic virus (BMV) is an RNA virus, and its three genomic RNAs are encapsidated in separate virions. Through proteomic, structural, and biochemical analyses, this work shows that posttranslational modifications, specifically, phosphorylation, on the capsid protein regulate the capsid-RNA interaction and the stability of the virions and affect viral gene expression. Mutational analysis confirmed that changes in modification affected virion stability and the timing of viral infection. The mechanism for modification of the virion has striking parallels to the mechanism of regulation of chromatin packaging by nucleosomes.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures




Similar articles
-
Unravelling the Stability and Capsid Dynamics of the Three Virions of Brome Mosaic Virus Assembled Autonomously In Vivo.J Virol. 2020 Mar 31;94(8):e01794-19. doi: 10.1128/JVI.01794-19. Print 2020 Mar 31. J Virol. 2020. PMID: 31996436 Free PMC article.
-
The plant host can affect the encapsidation of brome mosaic virus (BMV) RNA: BMV virions are surprisingly heterogeneous.J Mol Biol. 2014 Mar 6;426(5):1061-76. doi: 10.1016/j.jmb.2013.09.007. Epub 2013 Sep 13. J Mol Biol. 2014. PMID: 24036424 Free PMC article.
-
The tripartite virions of the brome mosaic virus have distinct physical properties that affect the timing of the infection process.J Virol. 2014 Jun;88(11):6483-91. doi: 10.1128/JVI.00377-14. Epub 2014 Mar 26. J Virol. 2014. PMID: 24672042 Free PMC article.
-
The coat protein leads the way: an update on basic and applied studies with the Brome mosaic virus coat protein.Mol Plant Pathol. 2011 May;12(4):403-12. doi: 10.1111/j.1364-3703.2010.00678.x. Epub 2010 Nov 25. Mol Plant Pathol. 2011. PMID: 21453435 Free PMC article. Review.
-
Brome mosaic virus RNA replication: revealing the role of the host in RNA virus replication.Annu Rev Phytopathol. 2003;41:77-98. doi: 10.1146/annurev.phyto.41.052002.095717. Epub 2003 Mar 10. Annu Rev Phytopathol. 2003. PMID: 12651962 Review.
Cited by
-
Variability among the Isolates of Broad Bean Mottle Virus and Encapsidation of Host RNAs.Pathogens. 2022 Jul 21;11(7):817. doi: 10.3390/pathogens11070817. Pathogens. 2022. PMID: 35890061 Free PMC article.
-
The intrinsically disordered N-terminal arm of the brome mosaic virus coat protein specifically recognizes the RNA motif that directs the initiation of viral RNA replication.Nucleic Acids Res. 2018 Jan 9;46(1):324-335. doi: 10.1093/nar/gkx1087. Nucleic Acids Res. 2018. PMID: 29140480 Free PMC article.
-
Abscisic Acid Connects Phytohormone Signaling with RNA Metabolic Pathways and Promotes an Antiviral Response that Is Evaded by a Self-Controlled RNA Virus.Plant Commun. 2020 Sep 14;1(5):100099. doi: 10.1016/j.xplc.2020.100099. Epub 2020 Jul 7. Plant Commun. 2020. PMID: 32984814 Free PMC article.
-
Intrinsically-disordered N-termini in human parechovirus 1 capsid proteins bind encapsidated RNA.Sci Rep. 2018 Apr 11;8(1):5820. doi: 10.1038/s41598-018-23552-7. Sci Rep. 2018. PMID: 29643409 Free PMC article.
-
Structural and Biochemical Characterization of Endoribonuclease Nsp15 Encoded by Middle East Respiratory Syndrome Coronavirus.J Virol. 2018 Oct 29;92(22):e00893-18. doi: 10.1128/JVI.00893-18. Print 2018 Nov 15. J Virol. 2018. PMID: 30135128 Free PMC article.
References
-
- Domingo E, Escarmis C, Sevilla N, Moya A, Elena SF, Quer J, Novella IS, Holland JJ. 1996. Basic concepts in RNA virus evolution. FASEB J 10:859–864. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

