Designing, construction and characterization of genetically encoded FRET-based nanosensor for real time monitoring of lysine flux in living cells
- PMID: 27334743
- PMCID: PMC4917951
- DOI: 10.1186/s12951-016-0204-y
Designing, construction and characterization of genetically encoded FRET-based nanosensor for real time monitoring of lysine flux in living cells
Abstract
Background: Engineering microorganisms in order to improve the metabolite flux needs a detailed knowledge of the concentrations and flux rates of metabolites and metabolic intermediates in vivo. Fluorescence resonance energy transfer (FRET) based genetically encoded nanosensors represent a promising tool for measuring the metabolite levels and corresponding rate changes in live cells. Here, we report the development of a series of FRET based genetically encoded nanosensor for real time measurement of lysine at cellular level, as the improvement of microbial strains for the production of L-lysine is of major interest in industrial biotechnology.
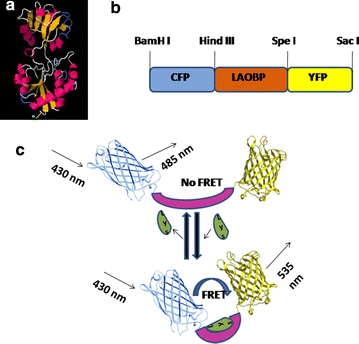
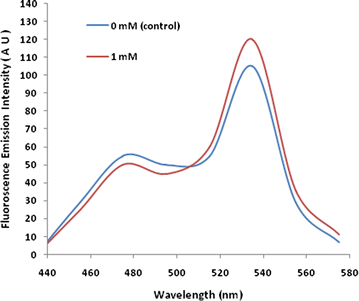
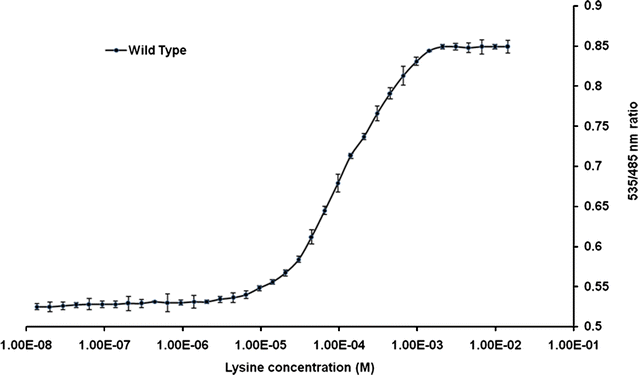
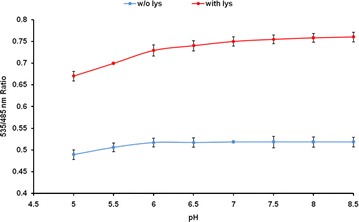
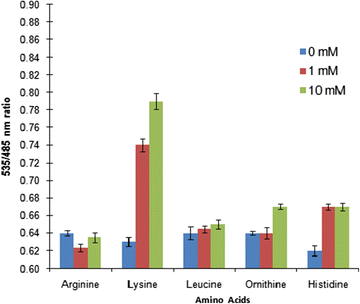
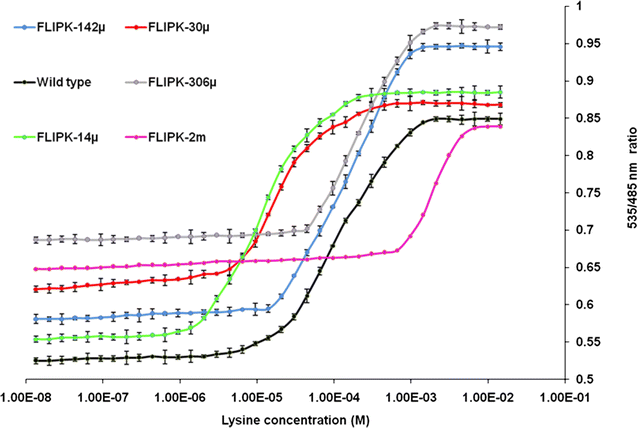
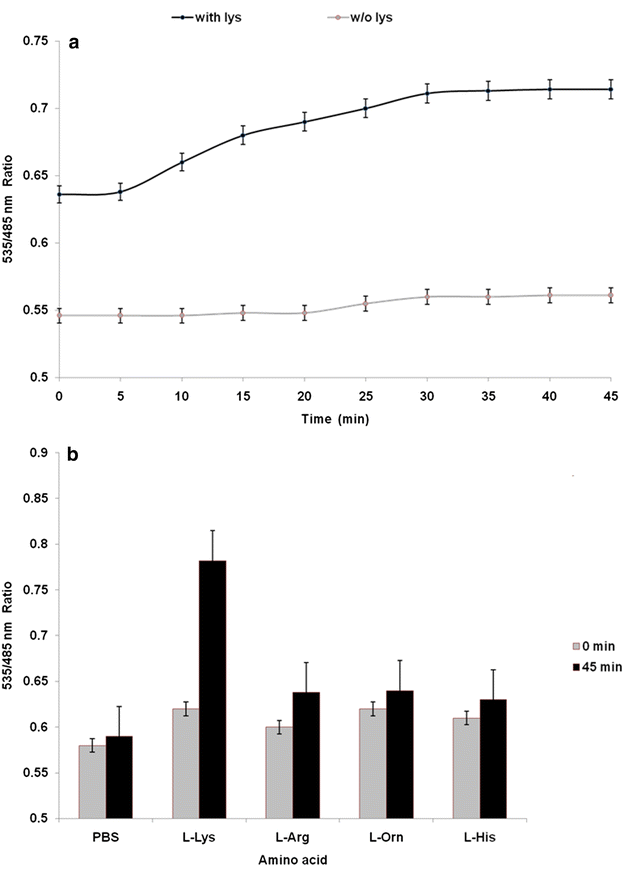
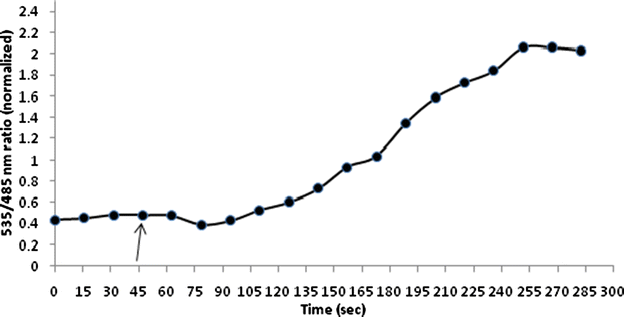
Results: The lysine binding periplasmic protein (LAO) from Salmonella enterica serovar typhimurium LT2 strain was used as the reporter element for the sensor. The LAO was sandwiched between GFP variants i.e. cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP). Affinity, pH stability, specificity and metal ions effects was scrutinized for the in vitro characterization of this nanosensor, named as FLIPK. The FLIPK is specific to lysine and found to be stable with the pH within the physiological range. The calculated affinity (K d ) of FLIPK was 97 µM. For physiological applications, mutants with different binding affinities were also generated and investigated in vitro. The developed nanosensor efficiently monitored the intracellular level of lysine in bacterial as well as yeast cell.
Conclusion: The developed novel lysine fluorescence resonance energy transfer sensors can be used for in vivo monitoring of lysine levels in prokaryotes as well as eukaryotes. The potential of these sensors is that they can be used as reporter tools in the development of metabolically engineered microbial strains or for real-time monitoring of intracellular lysine during fermentation.
Keywords: Fluorescent protein; Fluorescent resonance energy transfer (FRET); Genetically encoded nanosensor; Lysine; Periplasmic binding protein.
Figures

References
-
- Kimura E. l-Glutamate production. In: Eggeling L, Bott M, editors. Handbook of Cornybacterium glutamicum. Boca Raton: CRC Press; 2005. pp. 439–464.
-
- Hanbler E, Burkovski A. Molecular mechanisms of nitrogen control in corynebacteria. In: Burkovski A, editor. Corynebacteria: genomics and molecular biology. UK: Caister Academic Press, Norfolk; 2008. pp. 183–201.
-
- Eggeling L. Amino acids. In: Ratledge C, Kristiansen B, editors. Basic biotechnology. London: Cambridge University Press; 2001. pp. 281–303.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

