Calcineurin β protects brain after injury by activating the unfolded protein response
- PMID: 27334877
- PMCID: PMC4983525
- DOI: 10.1016/j.nbd.2016.06.011
Calcineurin β protects brain after injury by activating the unfolded protein response
Abstract
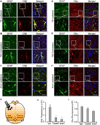
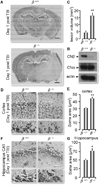
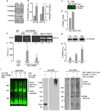
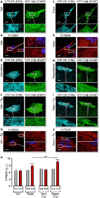
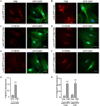
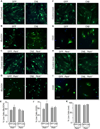
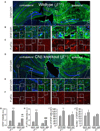
The Ca(2+)-dependent phosphatase, calcineurin (CN) is thought to play a detrimental role in damaged neurons; however, its role in astrocytes is unclear. In cultured astrocytes, CNβ expression increased after treatment with a sarco/endoplasmic reticulum Ca(2+)-ATPase inhibitor, thapsigargin, and with oxygen and glucose deprivation, an in vitro model of ischemia. Similarly, CNβ was induced in astrocytes in vivo in two different mouse models of brain injury - photothrombotic stroke and traumatic brain injury (TBI). Immunoprecipitation and chemical activation dimerization methods pointed to physical interaction of CNβ with the unfolded protein response (UPR) sensor, protein kinase RNA-like endoplasmic reticulum kinase (PERK). In accordance, induction of CNβ resulted in oligomerization and activation of PERK. Strikingly, the presence of a phosphatase inhibitor did not interfere with CNβ-mediated activation of PERK, suggesting a hitherto undiscovered non-enzymatic role for CNβ. Importantly, the cytoprotective function of CNβ was PERK-dependent both in vitro and in vivo. Loss of CNβ in vivo resulted in a significant increase in cerebral damage, and correlated with a decrease in astrocyte size, PERK activity and glial fibrillary acidic protein (GFAP) expression. Taken together, these data reveal a critical role for the CNβ-PERK axis in not only prolonging astrocyte cell survival but also in modulating astrogliosis after brain injury.
Keywords: Calcium; Endoplasmic reticulum; Ischemia; Stress; Traumatic brain injury.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Neurite atrophy and apoptosis mediated by PERK signaling after accumulation of GM2-ganglioside.Biochim Biophys Acta Mol Cell Res. 2019 Feb;1866(2):225-239. doi: 10.1016/j.bbamcr.2018.10.014. Epub 2018 Oct 30. Biochim Biophys Acta Mol Cell Res. 2019. PMID: 30389374
-
Calcineurin interacts with PERK and dephosphorylates calnexin to relieve ER stress in mammals and frogs.PLoS One. 2010 Aug 5;5(8):e11925. doi: 10.1371/journal.pone.0011925. PLoS One. 2010. PMID: 20700529 Free PMC article.
-
Protein kinase RNA-like endoplasmic reticulum kinase (PERK)/calcineurin signaling is a novel pathway regulating intracellular calcium accumulation which might be involved in ventricular arrhythmias in diabetic cardiomyopathy.Cell Signal. 2014 Dec;26(12):2591-600. doi: 10.1016/j.cellsig.2014.08.015. Epub 2014 Aug 22. Cell Signal. 2014. PMID: 25152364
-
[Research of eye diseases associated with PERK mediated endoplasmic reticulum stress].Zhonghua Yan Ke Za Zhi. 2012 Aug;48(8):759-62. Zhonghua Yan Ke Za Zhi. 2012. PMID: 23141519 Review. Chinese.
-
Coordination of stress, Ca2+, and immunogenic signaling pathways by PERK at the endoplasmic reticulum.Biol Chem. 2016 Jul 1;397(7):649-56. doi: 10.1515/hsz-2016-0108. Biol Chem. 2016. PMID: 26872313 Review.
Cited by
-
Neuronal-targeted TFEB rescues dysfunction of the autophagy-lysosomal pathway and alleviates ischemic injury in permanent cerebral ischemia.Autophagy. 2019 Mar;15(3):493-509. doi: 10.1080/15548627.2018.1531196. Epub 2018 Oct 18. Autophagy. 2019. PMID: 30304977 Free PMC article.
-
Ursodeoxycholic Acid Binds PERK and Ameliorates Neurite Atrophy in a Cellular Model of GM2 Gangliosidosis.Int J Mol Sci. 2023 Apr 13;24(8):7209. doi: 10.3390/ijms24087209. Int J Mol Sci. 2023. PMID: 37108372 Free PMC article.
-
Calcium and Non-Penetrating Traumatic Brain Injury: A Proposal for the Implementation of an Early Therapeutic Treatment for Initial Head Insults.Biomolecules. 2024 Jul 15;14(7):853. doi: 10.3390/biom14070853. Biomolecules. 2024. PMID: 39062567 Free PMC article. Review.
-
Synergistic effects of brain injury and aging: common mechanisms of proteostatic dysfunction.Trends Neurosci. 2021 Sep;44(9):728-740. doi: 10.1016/j.tins.2021.06.003. Epub 2021 Jul 20. Trends Neurosci. 2021. PMID: 34301397 Free PMC article. Review.
-
Prevention of brain damage after traumatic brain injury by pharmacological enhancement of KCNQ (Kv7, "M-type") K+ currents in neurons.J Cereb Blood Flow Metab. 2020 Jun;40(6):1256-1273. doi: 10.1177/0271678X19857818. Epub 2019 Jul 4. J Cereb Blood Flow Metab. 2020. PMID: 31272312 Free PMC article.
References
-
- Agostinho P, Lopes JP, Velez Z, Oliveira CR. Overactivation of calcineurin induced by amyloid-beta and prion proteins. Neurochem. Int. 2008 Mayy;52(6):1226–1233. PubMed PMID: 18295934) - PubMed
-
- Axten JM, Medina JR, Feng Y, Shu A, Romeril SP, Grant SW, et al. Discovery of 7-methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2,3-dihydro-1H–indol-5-yl)-7H–p yrrolo[2,3-d]pyrimidin-4-amine (GSK2606414), a potent and selective first-in-class inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK) J. Med. Chem. 2012 Aug 23;55(16):7193–7207. PubMed PMID: 22827572) - PubMed
-
- Badiola N, Penas C, Minano-Molina A, Barneda-Zahonero B, Fado R, Sanchez-Opazo G, et al. Induction of ER stress in response to oxygen-glucose deprivation of cortical cultures involves the activation of the PERK and IRE-1 pathways and of caspase-12. Cell Death Dis. 2011;2:e149. PubMed PMID: 21525936. Pubmed Central PMCID: 3122062. Epub 2011/04/29) - PMC - PubMed
-
- Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2000 Junn;2(6):326–332. PubMed PMID: 10854322. Epub 2000/06/15. eng) - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

