Identification of a Conserved RNA-dependent RNA Polymerase (RdRp)-RNA Interface Required for Flaviviral Replication
- PMID: 27334920
- PMCID: PMC5016140
- DOI: 10.1074/jbc.M116.724013
Identification of a Conserved RNA-dependent RNA Polymerase (RdRp)-RNA Interface Required for Flaviviral Replication
Abstract
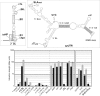
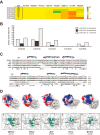
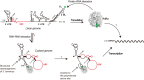
Dengue virus, an ∼10.7-kb positive-sense RNA virus, is the most common arthropod-communicated pathogen in the world. Despite dengue's clear epidemiological importance, mechanisms for its replication remain elusive. Here, we probed the entire dengue genome for interactions with viral RNA-dependent RNA polymerase (RdRp), and we identified the dominant interaction as a loop-forming ACAG motif in the 3' positive-stranded terminus, complicating the prevailing model of replication. A subset of interactions coincides with known flaviviral recombination sites inside the viral protein-coding region. Specific recognition of the RNA element occurs via an arginine patch in the C-terminal thumb domain of RdRp. We also show that the highly conserved nature of the consensus RNA motif may relate to its tolerance to various mutations in the interacting region of RdRp. Disruption of the interaction resulted in loss of viral replication ability in cells. This unique RdRp-RNA interface is found throughout flaviviruses, implying possibilities for broad disease interventions.
Keywords: RNA-dependent RNA polymerase (RdRp); dengue virus (DENV); untranslated region (UTR); viral non-structural protein (NS) 5; viral polymerase; viral protein; viral replication; virology; yeast three-hybrid (Y3H).
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




Similar articles
-
Two RNA Tunnel Inhibitors Bind in Highly Conserved Sites in Dengue Virus NS5 Polymerase: Structural and Functional Studies.J Virol. 2020 Nov 23;94(24):e01130-20. doi: 10.1128/JVI.01130-20. Print 2020 Nov 23. J Virol. 2020. PMID: 32907977 Free PMC article.
-
Interference of dengue replication by blocking the access of 3' SL RNA to the viral RNA-dependent RNA polymerase.Antiviral Res. 2020 Oct;182:104921. doi: 10.1016/j.antiviral.2020.104921. Epub 2020 Aug 22. Antiviral Res. 2020. PMID: 32835694
-
A crystal structure of the dengue virus non-structural protein 5 (NS5) polymerase delineates interdomain amino acid residues that enhance its thermostability and de novo initiation activities.J Biol Chem. 2013 Oct 25;288(43):31105-14. doi: 10.1074/jbc.M113.508606. Epub 2013 Sep 11. J Biol Chem. 2013. PMID: 24025331 Free PMC article.
-
The Transactions of NS3 and NS5 in Flaviviral RNA Replication.Adv Exp Med Biol. 2018;1062:147-163. doi: 10.1007/978-981-10-8727-1_11. Adv Exp Med Biol. 2018. PMID: 29845531 Review.
-
Flavivirus RNA-Dependent RNA Polymerase Interacts with Genome UTRs and Viral Proteins to Facilitate Flavivirus RNA Replication.Viruses. 2019 Oct 10;11(10):929. doi: 10.3390/v11100929. Viruses. 2019. PMID: 31658680 Free PMC article. Review.
Cited by
-
Specific Interaction of DDX6 with an RNA Hairpin in the 3' UTR of the Dengue Virus Genome Mediates G1 Phase Arrest.J Virol. 2021 Aug 10;95(17):e0051021. doi: 10.1128/JVI.00510-21. Epub 2021 Aug 10. J Virol. 2021. PMID: 34132569 Free PMC article.
-
Amino Acid Substitutions in NS5 Contribute Differentially to Tembusu Virus Attenuation in Ducklings and Cell Cultures.Viruses. 2021 May 16;13(5):921. doi: 10.3390/v13050921. Viruses. 2021. PMID: 34065634 Free PMC article.
-
NMR structure of Dengue West Nile viruses stem-loop B: A key cis-acting element for flavivirus replication.Biochem Biophys Res Commun. 2020 Oct 22;531(4):522-527. doi: 10.1016/j.bbrc.2020.07.115. Epub 2020 Aug 15. Biochem Biophys Res Commun. 2020. PMID: 32807496 Free PMC article.
-
Preventing the development of severe COVID-19 by modifying immunothrombosis.Life Sci. 2021 Jan 1;264:118617. doi: 10.1016/j.lfs.2020.118617. Epub 2020 Oct 20. Life Sci. 2021. PMID: 33096114 Free PMC article. Review.
-
AT-752, a double prodrug of a guanosine nucleotide analog, inhibits yellow fever virus in a hamster model.PLoS Negl Trop Dis. 2022 Jan 24;16(1):e0009937. doi: 10.1371/journal.pntd.0009937. eCollection 2022 Jan. PLoS Negl Trop Dis. 2022. PMID: 35073319 Free PMC article.
References
-
- Dreher T. W., and Hall T. C. (1988) Mutational analysis of the sequence and structural requirements in brome mosaic virus RNA for minus strand promoter activity. J. Mol. Biol. 201, 31–40 - PubMed
-
- Song C., and Simon A. E. (1995) Requirement of a 3′-terminal stem-loop in in vitro transcription by an RNA-dependent RNA polymerase. J. Mol. Biol. 254, 6–14 - PubMed
-
- Oh J. W., Sheu G. T., and Lai M. M. (2000) Template requirement and initiation site selection by hepatitis C virus polymerase on a minimal viral RNA template. J. Biol. Chem. 275, 17710–17717 - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

