Salivary total α-synuclein, oligomeric α-synuclein and SNCA variants in Parkinson's disease patients
- PMID: 27335051
- PMCID: PMC4917865
- DOI: 10.1038/srep28143
Salivary total α-synuclein, oligomeric α-synuclein and SNCA variants in Parkinson's disease patients
Abstract
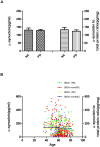
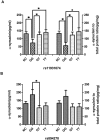
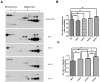
The present study was to evaluate the diagnostic value of salivary total and oligomeric α-synuclein levels in PD. Furthermore, we sought to explore the relationship between salivary total α-synuclein and α-synuclein SNP variants levels. 201 PD patients and 67 controls were recruited, of which there also had the genetic information of two positive α-synuclein (SNCA) loci. Salivary total α-synuclein was assayed using a highly sensitive Luminex assay and oligomeric α-synuclein was quantified by the combination of Gel filtration chromatography and Western blot, respectively. From our analysis,No difference in salivary total α-synuclein levels was found between PD patients and healthy controls, it decreased with age in PD patients, and was closely associated with genotypic distribution of rs11931074 and rs894278 in PD, respectively. After controlled for age and genders, G allele of rs11931074 was correlated with lower salivary total α-synuclein levels, while G allele of rs894278 was also correlated with the higher levels. Simultaneously, the further study was shown that salivary oligomeric α-synuclein in PD patients significantly increased comparing to healthy controls. In conclusions,our study firstly demonstrated that salivary total α-synuclein levels could be manipulated by different α-synuclein SNPs and salivary oligomeric α-synuclein could be a potential diagnostic indicator of PD.
Figures
Similar articles
-
SNCA variants and alpha-synuclein level in CD45+ blood cells in Parkinson's disease.J Neurol Sci. 2018 Dec 15;395:135-140. doi: 10.1016/j.jns.2018.10.002. Epub 2018 Oct 3. J Neurol Sci. 2018. PMID: 30316070
-
Association of SNCA variants with α-synuclein of gastric and colonic mucosa in Parkinson's disease.Parkinsonism Relat Disord. 2019 Apr;61:151-155. doi: 10.1016/j.parkreldis.2018.10.028. Epub 2018 Oct 26. Parkinsonism Relat Disord. 2019. PMID: 30424941
-
Cerebellar alpha-synuclein levels are decreased in Parkinson's disease and do not correlate with SNCA polymorphisms associated with disease in a Swedish material.FASEB J. 2008 Oct;22(10):3509-14. doi: 10.1096/fj.08-110148. Epub 2008 Jul 7. FASEB J. 2008. PMID: 18606870
-
Salivary alpha-synuclein as a biomarker for Parkinson's disease: a systematic review.J Neural Transm (Vienna). 2019 Nov;126(11):1373-1382. doi: 10.1007/s00702-019-02062-4. Epub 2019 Aug 10. J Neural Transm (Vienna). 2019. PMID: 31401695
-
Association between alpha-synuclein (SNCA) rs11931074 variability and susceptibility to Parkinson's disease: an updated meta-analysis of 41,811 patients.Neurol Sci. 2020 Feb;41(2):271-280. doi: 10.1007/s10072-019-04107-8. Epub 2019 Nov 22. Neurol Sci. 2020. PMID: 31758346 Review.
Cited by
-
The Role of Salivary Biomarkers in the Early Diagnosis of Alzheimer's Disease and Parkinson's Disease.Diagnostics (Basel). 2021 Feb 22;11(2):371. doi: 10.3390/diagnostics11020371. Diagnostics (Basel). 2021. PMID: 33671562 Free PMC article. Review.
-
Prospect of Alpha-Synuclein (A-Syn) Isolation From Saliva as a Promising Diagnostic Biomarker Alternative in Parkinson's Disease (PD): A Systematic Review.Cureus. 2022 Oct 3;14(10):e29880. doi: 10.7759/cureus.29880. eCollection 2022 Oct. Cureus. 2022. PMID: 36348879 Free PMC article. Review.
-
Salivary α-Synuclein as a Candidate Biomarker of Parkinsonism in 22q11.2 Deletion Syndrome.Mov Disord Clin Pract. 2024 Jul;11(7):808-813. doi: 10.1002/mdc3.14046. Epub 2024 Apr 25. Mov Disord Clin Pract. 2024. PMID: 38661486 Free PMC article.
-
Common SNCA Genetic Variants and Parkinson's Disease Risk: A Systematic Review and Meta-Analysis.Int J Mol Sci. 2025 Jun 23;26(13):6001. doi: 10.3390/ijms26136001. Int J Mol Sci. 2025. PMID: 40649779 Free PMC article. Review.
-
Salivary alpha-synuclein as a potential fluid biomarker in Parkinson's disease: A systematic review and meta-analysis.Aging Med (Milton). 2022 Jan 24;5(1):53-62. doi: 10.1002/agm2.12192. eCollection 2022 Mar. Aging Med (Milton). 2022. PMID: 35309157 Free PMC article.
References
-
- Halliday G., Lees A. & Stern M. Milestones in Parkinson’s disease–clinical and pathologic features. Mov Disord. 26(6), 1015–21 (2011). - PubMed
-
- El-Agnaf O. M. et al. Detection of oligomeric forms of alpha-synuclein protein in human plasma as a potential biomarker for Parkinson’s disease. FASEB J. 20(3), 419–25 (2006). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

