A Feasibility Study of Moxibustion for Treating Anorexia and Improving Quality of Life in Patients With Metastatic Cancer: A Randomized Sham-Controlled Trial
- PMID: 27335088
- PMCID: PMC5736070
- DOI: 10.1177/1534735416654762
A Feasibility Study of Moxibustion for Treating Anorexia and Improving Quality of Life in Patients With Metastatic Cancer: A Randomized Sham-Controlled Trial
Abstract
Objective: The aim of this study was to determine the feasibility, acceptability, and safety of using moxibustion for treating anorexia and improving quality of life in patients with metastatic cancer.
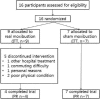
Methods: We conducted a randomized sham-controlled trial of moxibustion. Sixteen patients with metastatic cancer were recruited from Daejeon, South Korea. The patients were randomly placed into a true or a sham moxibustion group and received 10 true or sham moxibustion treatments administered to the abdomen (CV12, CV8, CV4) and legs (ST36) over a 2-week period. Outcome measures included interest in participating in the trial, identification of successful recruitment strategies, the appropriateness of eligibility criteria, and compliance with the treatment plan (ie, attendance at treatment sessions). Clinical outcomes included results of the Functional Assessment of Anorexia/Cachexia Therapy (FAACT), answers on the European Organization for Research and Treatment of Cancer 30-item core quality of life (EORTC QLQ-C30) questionnaires, scores on the visual analogue scale (VAS), and the results from blood tests and a safety evaluation.
Results: Moxibustion was an acceptable intervention in patients with metastatic cancer. Compliance with the treatment protocol was high, with 11 patients completing all 10 treatments. No serious adverse events related to moxibustion occurred, but 4 patients in the true moxibustion group reported mild rubefaction, which disappeared in a few hours.
Conclusion: This study suggests that moxibustion may be safely used to treat anorexia and improve quality of life in patients with metastatic cancer. However, further research is needed to confirm this result.
Keywords: anorexia; feasibility study; metastatic cancer; moxibustion; quality of life.
Conflict of interest statement
Figures


Similar articles
-
Efficacy and safety of Yukgunja-Tang for treating anorexia in patients with cancer: The protocol for a pilot, randomized, controlled trial.Medicine (Baltimore). 2019 Oct;98(40):e16950. doi: 10.1097/MD.0000000000016950. Medicine (Baltimore). 2019. PMID: 31577697 Free PMC article.
-
Moxibustion for cancer-related fatigue: study protocol for a randomized controlled trial.BMC Complement Altern Med. 2017 Jul 5;17(1):353. doi: 10.1186/s12906-017-1856-3. BMC Complement Altern Med. 2017. PMID: 28679410 Free PMC article. Clinical Trial.
-
[Heat-sensitive moxibustion assisted in palliative treatment to improve the quality of life in elderly patients with malignant tumor: a randomized controlled trial].Zhongguo Zhen Jiu. 2025 Feb 12;45(2):167-72. doi: 10.13703/j.0255-2930.20240403-k0002. Zhongguo Zhen Jiu. 2025. PMID: 39943757 Clinical Trial. Chinese.
-
Acupuncture for Cancer-Related Anorexia: a Review of the Current Evidence.Curr Oncol Rep. 2021 May 5;23(7):82. doi: 10.1007/s11912-021-01067-1. Curr Oncol Rep. 2021. PMID: 33948746
-
Appetite problem in cancer patients: Pathophysiology, diagnosis, and treatment.Cancer Treat Res Commun. 2021;27:100336. doi: 10.1016/j.ctarc.2021.100336. Epub 2021 Feb 13. Cancer Treat Res Commun. 2021. PMID: 33607591 Review.
Cited by
-
Dropouts in randomized clinical trials of Korean medicine interventions: a systematic review and meta-analysis.Trials. 2021 Mar 1;22(1):176. doi: 10.1186/s13063-021-05114-x. Trials. 2021. PMID: 33648566 Free PMC article.
-
The efficacy and safety of moxibustion for chemotherapy-induced gastrointestinal adverse reaction: A protocol for systematic review and meta-analysis.Medicine (Baltimore). 2020 Aug 28;99(35):e22042. doi: 10.1097/MD.0000000000022042. Medicine (Baltimore). 2020. PMID: 32871961 Free PMC article.
-
Effectiveness, safety, and economic evaluation of adjuvant moxibustion therapy for aromatase inhibitor-induced arthralgia of postmenopausal breast cancer stage I to III patients: Study protocol for a prospective, randomized, assessor-blind, usual-care controlled, parallel-group, pilot clinical trial.Medicine (Baltimore). 2019 Sep;98(38):e17260. doi: 10.1097/MD.0000000000017260. Medicine (Baltimore). 2019. PMID: 31568000 Free PMC article. Clinical Trial.
-
Effect of Moxibustion Combined With Cisplatin on Tumor Microenvironment Hypoxia and Vascular Normalization in Lewis Lung Cancer Mice.Integr Cancer Ther. 2023 Jan-Dec;22:15347354231198195. doi: 10.1177/15347354231198195. Integr Cancer Ther. 2023. PMID: 37694878 Free PMC article.
-
Moxibustion for treating cancer-related fatigue: A multicenter, assessor-blinded, randomized controlled clinical trial.Cancer Med. 2021 Jul;10(14):4721-4733. doi: 10.1002/cam4.4020. Epub 2021 Jun 29. Cancer Med. 2021. PMID: 34189864 Free PMC article. Clinical Trial.
References
-
- Lao L. Traditional Chinese medicine: part III. In: Jonas WB, Levin JS. eds. Essentials of Complementary and Alternative Medicine. Philadelphia, PA: Lippincott Williams & Wilkins; 1999:219-232.
-
- Yamashita H, Ichiman Y, Tanno Y. Changes in peripheral lymphocyte subpopulations after direct moxibustion. Am J Chin Med. 2001;29:227-235. - PubMed
-
- Im ST, Kim KH, Kim KS. A study of physical characteristics of moxibustion. J Korean Acupunt Moxibust Soc. 1994;11:327-336.
-
- Cho WC. Acupuncture and Moxibustion as an Evidence-Based Therapy for Cancer. New York, NY: Springer; 2012.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

