Efficacy and safety of brivaracetam for partial-onset seizures in 3 pooled clinical studies
- PMID: 27335114
- PMCID: PMC4955277
- DOI: 10.1212/WNL.0000000000002864
Efficacy and safety of brivaracetam for partial-onset seizures in 3 pooled clinical studies
Abstract
Objective: To assess the efficacy, safety, and tolerability of adjunctive brivaracetam (BRV), a selective, high-affinity ligand for SV2A, for treatment of partial-onset (focal) seizures (POS) in adults.
Methods: Data were pooled from patients (aged 16-80 years) with POS uncontrolled by 1 to 2 antiepileptic drugs receiving BRV 50, 100, or 200 mg/d or placebo, without titration, in 3 phase III studies of BRV (NCT00490035, NCT00464269, and NCT01261325, ClinicalTrials.gov, funded by UCB Pharma). The studies had an 8-week baseline and a 12-week treatment period. Patients receiving concomitant levetiracetam were excluded from the efficacy pool.
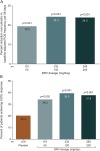
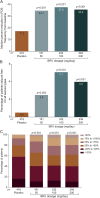
Results: In the efficacy population (n = 1,160), reduction over placebo (95% confidence interval) in baseline-adjusted POS frequency/28 days was 19.5% (8.0%-29.6%) for 50 mg/d (p = 0.0015), 24.4% (16.8%-31.2%) for 100 mg/d (p < 0.00001), and 24.0% (15.3%-31.8%) for 200 mg/d (p < 0.00001). The ≥50% responder rate was 34.2% (50 mg/d, p = 0.0015), 39.5% (100 mg/d, p < 0.00001), and 37.8% (200 mg/d, p = 0.00003) vs 20.3% for placebo (p < 0.01). Across the safety population groups (n = 1,262), 90.0% to 93.9% completed the studies. Treatment-emergent adverse events (TEAEs) were reported by 68.0% BRV overall (n = 803) and 62.1% placebo (n = 459). Serious TEAEs were reported by 3.0% (BRV) and 2.8% (placebo); 3 patients receiving BRV and one patient receiving placebo died. TEAEs in ≥5% patients taking BRV (vs placebo) were somnolence (15.2% vs 8.5%), dizziness (11.2% vs 7.2%), headache (9.6% vs 10.2%), and fatigue (8.7% vs 3.7%).
Conclusions: Adjunctive BRV was effective and generally well tolerated in adults with POS.
Classification of evidence: This analysis provides Class I evidence that adjunctive BRV is effective in reducing POS frequency in adults with epilepsy and uncontrolled seizures.
© 2016 American Academy of Neurology.
Figures
Comment in
-
Letter re: Efficacy and safety of brivaracetam for partial-onset seizures in 3 pooled clinical studies.Neurology. 2017 Oct 17;89(16):1756. doi: 10.1212/WNL.0000000000004512. Neurology. 2017. PMID: 29038138 No abstract available.
References
-
- Löscher W, Schmidt D. Modern antiepileptic drug development has failed to deliver: ways out of the current dilemma. Epilepsia 2011;52:657–678. - PubMed
-
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med 2000;342:314–319. - PubMed
-
- Xu T, Bajjalieh SM. SV2 modulates the size of the readily releasable pool of secretory vesicles. Nat Cell Biol 2001;3:691–698. - PubMed
-
- Mendoza-Torreblanca JG, Vanoye-Carlo A, Phillips-Farfan BV, Carmona-Aparicio L, Gomez-Lira G. Synaptic vesicle protein 2A: basic facts and role in synaptic function. Eur J Neurosci 2013;38:3529–3539. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
