Systematic tracking of altered haematopoiesis during sporozoite-mediated malaria development reveals multiple response points
- PMID: 27335321
- PMCID: PMC4929935
- DOI: 10.1098/rsob.160038
Systematic tracking of altered haematopoiesis during sporozoite-mediated malaria development reveals multiple response points
Abstract
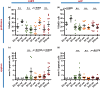
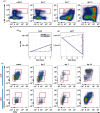
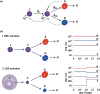
Haematopoiesis is the complex developmental process that maintains the turnover of all blood cell lineages. It critically depends on the correct functioning of rare, quiescent haematopoietic stem cells (HSCs) and more numerous, HSC-derived, highly proliferative and differentiating haematopoietic progenitor cells (HPCs). Infection is known to affect HSCs, with severe and chronic inflammatory stimuli leading to stem cell pool depletion, while acute, non-lethal infections exert transient and even potentiating effects. Both whether this paradigm applies to all infections and whether the HSC response is the dominant driver of the changes observed during stressed haematopoiesis remain open questions. We use a mouse model of malaria, based on natural, sporozoite-driven Plasmodium berghei infection, as an experimental platform to gain a global view of haematopoietic perturbations during infection progression. We observe coordinated responses by the most primitive HSCs and multiple HPCs, some starting before blood parasitaemia is detected. We show that, despite highly variable inter-host responses, primitive HSCs become highly proliferative, but mathematical modelling suggests that this alone is not sufficient to significantly impact the whole haematopoietic cascade. We observe that the dramatic expansion of Sca-1(+) progenitors results from combined proliferation of direct HSC progeny and phenotypic changes in downstream populations. We observe that the simultaneous perturbation of HSC/HPC population dynamics is coupled with early signs of anaemia onset. Our data uncover a complex relationship between Plasmodium and its host's haematopoiesis and raise the question whether the variable responses observed may affect the outcome of the infection itself and its long-term consequences on the host.
Keywords: haematopoietic stem cells; plasmodium infection; population dynamics.
© 2016 The Authors.
Figures

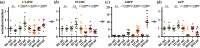

References
-
- Wilson A, et al. 2008. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135, 1118–1129. (doi:10.1016/j.cell.2008.10.048) - DOI - PubMed
-
- Gordon MY, Lewis JL, Marley SB. 2002. Of mice and men…and elephants. Blood 100, 4679–4680. (doi:10.1182/blood-2002-08-2517) - DOI - PubMed
-
- King KY, Goodell MA. 2011. Inflammatory modulation of HSCs: viewing the HSC as a foundation for the immune response. Nat. Rev. Immunol. 11, 685–692. (doi:10.1038/nri3062) - DOI - PMC - PubMed
-
- Mirantes C, Passegue E, Pietras EM. 2014. Pro-inflammatory cytokines: emerging players regulating HSC function in normal and diseased hematopoiesis. Exp. Cell Res. 329, 248–254. (doi:10.1016/j.yexcr.2014.08.017) - DOI - PMC - PubMed
-
- Baldridge MT, King KY, Goodell MA. 2011. Inflammatory signals regulate hematopoietic stem cells. Trends Immunol. 32, 57–65. (doi:10.1016/j.it.2010.12.003) - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

