Synapsin Isoforms Regulating GABA Release from Hippocampal Interneurons
- PMID: 27335405
- PMCID: PMC6601744
- DOI: 10.1523/JNEUROSCI.0011-16.2016
Synapsin Isoforms Regulating GABA Release from Hippocampal Interneurons
Abstract
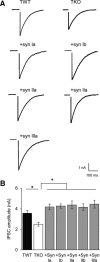
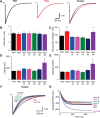
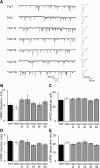
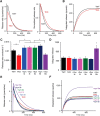
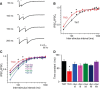
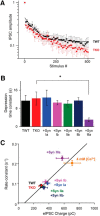
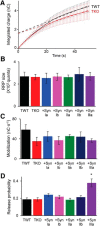
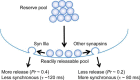
Although synapsins regulate GABA release, it is unclear which synapsin isoforms are involved. We identified the synapsin isoforms that regulate GABA release via rescue experiments in cultured hippocampal neurons from synapsin I, II, and III triple knock-out (TKO) mice. In situ hybridization indicated that five different synapsin isoforms are expressed in hippocampal interneurons. Evoked IPSC amplitude was reduced in TKO neurons compared with triple wild-type neurons and was rescued by introducing any of the five synapsin isoforms. This contrasts with hippocampal glutamatergic terminals, where only synapsin IIa rescues the TKO phenotype. Deconvolution analysis indicated that the duration of GABA release was prolonged in TKO neurons and this defect in release kinetics was rescued by each synapsin isoform, aside from synapsin IIIa. Because release kinetics remained slow, whereas peak release rate was rescued, there was a 2-fold increase in GABA release in TKO neurons expressing synapsin IIIa. TKO neurons expressing individual synapsin isoforms showed normal depression kinetics aside from more rapid depression in neurons expressing synapsin IIIa. Measurements of the cumulative amount of GABA released during repetitive stimulation revealed that the rate of mobilization of vesicles from the reserve pool to the readily releasable pool and the size of the readily releasable pool of GABAergic vesicles were unaffected by synapsins. Instead, synapsins regulate release of GABA from the readily releasable pool, with all isoforms aside from synapsin IIIa controlling release synchrony. These results indicate that synapsins play fundamentally distinct roles at different types of presynaptic terminals.
Significance statement: Synapsins are a family of proteins that regulate synaptic vesicle (SV) trafficking within nerve terminals. Here, we demonstrate that release of the inhibitory neurotransmitter GABA is supported by many different synapsin types. This contrasts with the release of other neurotransmitters, which typically is supported by only one type of synapsin. We also found that synapsins serve to synchronize the release of GABA in response to presynaptic action potentials, which is different from the synapsin-dependent trafficking of SVs in other nerve terminals. Our results establish that different synapsins play fundamentally different roles at nerve terminals releasing different types of neurotransmitters. This is an important clue to understanding how neurons release their neurotransmitters, a process essential for normal brain function.
Keywords: GABA; exocytosis; hippocampus; interneurons; synapsin; synaptic vesicle trafficking.
Copyright © 2016 the authors 0270-6474/16/366742-16$15.00/0.
Figures

Similar articles
-
Synapsin II Regulation of GABAergic Synaptic Transmission Is Dependent on Interneuron Subtype.J Neurosci. 2017 Feb 15;37(7):1757-1771. doi: 10.1523/JNEUROSCI.0844-16.2016. Epub 2017 Jan 13. J Neurosci. 2017. PMID: 28087765 Free PMC article.
-
Synapsin selectively controls the mobility of resting pool vesicles at hippocampal terminals.J Neurosci. 2012 Mar 21;32(12):3969-80. doi: 10.1523/JNEUROSCI.5058-11.2012. J Neurosci. 2012. PMID: 22442064 Free PMC article.
-
Synapsin-dependent reserve pool of synaptic vesicles supports replenishment of the readily releasable pool under intense synaptic transmission.Eur J Neurosci. 2012 Oct;36(8):3005-20. doi: 10.1111/j.1460-9568.2012.08225.x. Epub 2012 Jul 17. Eur J Neurosci. 2012. PMID: 22805168
-
Synapsin Isoforms and Synaptic Vesicle Trafficking.Mol Cells. 2015 Nov;38(11):936-40. doi: 10.14348/molcells.2015.0233. Epub 2015 Nov 20. Mol Cells. 2015. PMID: 26627875 Free PMC article. Review.
-
Synapsins and the Synaptic Vesicle Reserve Pool: Floats or Anchors?Cells. 2021 Mar 16;10(3):658. doi: 10.3390/cells10030658. Cells. 2021. PMID: 33809712 Free PMC article. Review.
Cited by
-
Short-term plasticity at cerebellar granule cell to molecular layer interneuron synapses expands information processing.Elife. 2019 May 13;8:e41586. doi: 10.7554/eLife.41586. Elife. 2019. PMID: 31081751 Free PMC article.
-
Dynamic Foot Stimulations During Short-Term Hindlimb Unloading Prevent Dysregulation of the Neurotransmission in the Hippocampus of Rats.Cell Mol Neurobiol. 2021 Oct;41(7):1549-1561. doi: 10.1007/s10571-020-00922-2. Epub 2020 Jul 18. Cell Mol Neurobiol. 2021. PMID: 32683580 Free PMC article.
-
Synapsin II Regulation of GABAergic Synaptic Transmission Is Dependent on Interneuron Subtype.J Neurosci. 2017 Feb 15;37(7):1757-1771. doi: 10.1523/JNEUROSCI.0844-16.2016. Epub 2017 Jan 13. J Neurosci. 2017. PMID: 28087765 Free PMC article.
-
The readily-releasable pool dynamically regulates multivesicular release.Elife. 2019 Jul 31;8:e47434. doi: 10.7554/eLife.47434. Elife. 2019. PMID: 31364987 Free PMC article.
-
The role of network connectivity on epileptiform activity.Sci Rep. 2021 Oct 21;11(1):20792. doi: 10.1038/s41598-021-00283-w. Sci Rep. 2021. PMID: 34675264 Free PMC article.
References
-
- Banker G, Goslin K. Culturing nerve cells. Cambridge, MA: MIT; 1984.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
