Cell-Type-Specific Modulation of Sensory Responses in Olfactory Bulb Circuits by Serotonergic Projections from the Raphe Nuclei
- PMID: 27335411
- PMCID: PMC4916254
- DOI: 10.1523/JNEUROSCI.3667-15.2016
Cell-Type-Specific Modulation of Sensory Responses in Olfactory Bulb Circuits by Serotonergic Projections from the Raphe Nuclei
Abstract
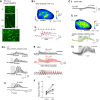
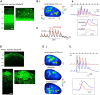
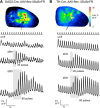
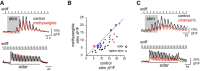
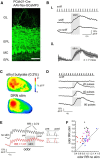
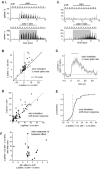
Serotonergic neurons in the brainstem raphe nuclei densely innervate the olfactory bulb (OB), where they can modulate the initial representation and processing of olfactory information. Serotonergic modulation of sensory responses among defined OB cell types is poorly characterized in vivo Here, we used cell-type-specific expression of optical reporters to visualize how raphe stimulation alters sensory responses in two classes of GABAergic neurons of the mouse OB glomerular layer, periglomerular (PG) and short axon (SA) cells, as well as mitral/tufted (MT) cells carrying OB output to piriform cortex. In PG and SA cells, brief (1-4 s) raphe stimulation elicited a large increase in the magnitude of responses linked to inhalation of ambient air, as well as modest increases in the magnitude of odorant-evoked responses. Near-identical effects were observed when the optical reporter of glutamatergic transmission iGluSnFR was expressed in PG and SA cells, suggesting enhanced excitatory input to these neurons. In contrast, in MT cells imaged from the dorsal OB, raphe stimulation elicited a strong increase in resting GCaMP fluorescence with only a slight enhancement of inhalation-linked responses to odorant. Finally, optogenetically stimulating raphe serotonergic afferents in the OB had heterogeneous effects on presumptive MT cells recorded extracellularly, with an overall modest increase in resting and odorant-evoked responses during serotonergic afferent stimulation. These results suggest that serotonergic afferents from raphe dynamically modulate olfactory processing through distinct effects on multiple OB targets, and may alter the degree to which OB output is shaped by inhibition during behavior.
Significance statement: Modulation of the circuits that process sensory information can profoundly impact how information about the external world is represented and perceived. This study investigates how the serotonergic system modulates the initial processing of olfactory information by the olfactory bulb, an obligatory relay between sensory neurons and cortex. We find that serotonergic projections from the raphe nuclei to the olfactory bulb dramatically enhance the responses of two classes of inhibitory interneurons to sensory input, that this effect is mediated by increased glutamatergic drive onto these neurons, and that serotonergic afferent activation alters the responses of olfactory bulb output neurons in vivo These results elucidate pathways by which neuromodulatory systems can dynamically regulate brain circuits during behavior.
Keywords: imaging; inhibition; neuromodulation; optogenetics; serotonin; sniffing.
Copyright © 2016 the authors 0270-6474/16/366820-16$15.00/0.
Figures


References
-
- Akerboom J, Chen TW, Wardill TJ, Tian L, Marvin JS, Mutlu S, Calderón NC, Esposti F, Borghuis BG, Sun XR, Gordus A, Orger MB, Portugues R, Engert F, Macklin JJ, Filosa A, Aggarwal A, Kerr RA, Takagi R, Kracun S, et al. Optimization of a GCaMP calcium indicator for neural activity imaging. J Neurosci. 2012;32:13819–13840. doi: 10.1523/JNEUROSCI.2601-12.2012. - DOI - PMC - PubMed
-
- Banerjee A, Marbach F, Anselmi F, Koh MS, Davis MB, Garcia da Silva P, Delevich K, Oyibo HK, Gupta P, Li B, Albeanu DF. An interglomerular circuit gates glomerular output and implements gain control in the mouse olfactory bulb. Neuron. 2015;87:193–207. doi: 10.1016/j.neuron.2015.06.019. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
