The Function of V-ATPases in Cancer
- PMID: 27335445
- PMCID: PMC4982037
- DOI: 10.1152/physrev.00035.2015
The Function of V-ATPases in Cancer
Abstract
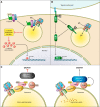
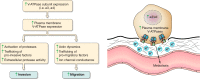
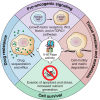
The vacuolar ATPases (V-ATPases) are a family of proton pumps that couple ATP hydrolysis to proton transport into intracellular compartments and across the plasma membrane. They function in a wide array of normal cellular processes, including membrane traffic, protein processing and degradation, and the coupled transport of small molecules, as well as such physiological processes as urinary acidification and bone resorption. The V-ATPases have also been implicated in a number of disease processes, including viral infection, renal disease, and bone resorption defects. This review is focused on the growing evidence for the important role of V-ATPases in cancer. This includes functions in cellular signaling (particularly Wnt, Notch, and mTOR signaling), cancer cell survival in the highly acidic environment of tumors, aiding the development of drug resistance, as well as crucial roles in tumor cell invasion, migration, and metastasis. Of greatest excitement is evidence that at least some tumors express isoforms of V-ATPase subunits whose disruption is not lethal, leading to the possibility of developing anti-cancer therapeutics that selectively target V-ATPases that function in cancer cells.
Copyright © 2016 the American Physiological Society.
Figures



References
-
- Adams DS, Masi A, Levin M. H+ pump-dependent changes in membrane voltage are an early mechanism necessary and sufficient to induce Xenopus tail regeneration. Development 134: 1323–1335, 2007. - PubMed
-
- Alwan HAJ, van Zoelen EJJ, van Leeuwen JEM. Ligand-induced lysosomal epidermal growth factor receptor (EGFR) degradation is preceded by proteasome-dependent EGFR de-ubiquitination. J Biol Chem 278: 35781–35790, 2003. - PubMed
-
- Alzamora R, Thali RF, Gong F, Smolak C, Li H, Baty CJ, Bertrand CA, Auchli Y, Brunisholz RA, Neumann D, Hallows KR, Pastor-Soler NM. PKA regulates vacuolar H+-ATPase localization and activity via direct phosphorylation of the a subunit in kidney cells. J Biol Chem 285: 24676–24685, 2010. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

