Cyanidin suppresses amyloid beta-induced neurotoxicity by inhibiting reactive oxygen species-mediated DNA damage and apoptosis in PC12 cells
- PMID: 27335564
- PMCID: PMC4904471
- DOI: 10.4103/1673-5374.182707
Cyanidin suppresses amyloid beta-induced neurotoxicity by inhibiting reactive oxygen species-mediated DNA damage and apoptosis in PC12 cells
Abstract
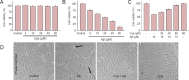
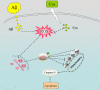
Amyloid beta (Aβ)-induced oxidative stress is a major pathologic hallmark of Alzheimer's disease. Cyanidin, a natural flavonoid compound, is neuroprotective against oxidative damage-mediated degeneration. However, its molecular mechanism remains unclear. Here, we investigated the effects of cyanidin pretreatment against Aβ-induced neurotoxicity in PC12 cells, and explored the underlying mechanisms. Cyanidin pretreatment significantly attenuated Aβ-induced cell mortality and morphological changes in PC12 cells. Mechanistically, cyanidin effectively blocked apoptosis induced by Aβ, by restoring the mitochondrial membrane potential via upregulation of Bcl-2 protein expression. Moreover, cyanidin markedly protected PC12 cells from Aβ-induced DNA damage by blocking reactive oxide species and superoxide accumulation. These results provide evidence that cyanidin suppresses Aβ-induced cytotoxicity, by preventing oxidative damage mediated by reactive oxide species, which in turn inhibits mitochondrial apoptosis. Our study demonstrates the therapeutic potential of cyanidin in the prevention of oxidative stress-mediated Aβ neurotoxicity.
Keywords: amyloid-beta; apoptosis; cyanidin; nerve regeneration; neural regeneration; oxidative damage; reactive oxide species.
Conflict of interest statement
Figures



References
-
- Acquaviva R, Russo A, Galvano F, Galvano G, Barcellona ML, Li Volti G. Cyanidin and cyanidin 3-O-beta-D-glucoside as DNA cleavage protectors and antioxidants. Cell Biol Toxicol. 2003;19:243–252. - PubMed
-
- Arai M, Shibata Y, Pugdee K, Abiko Y, Ogata Y. Effects of reactive oxygen species (ROS) on antioxidant system and osteoblastic differentiation in MC3T3-E1 cells. IUBMB Life. 2007;59:27–33. - PubMed
-
- Chun OK, Kim DO, Lee CY. Superoxide radical scavenging activity of the major polyphenols in fresh plums. J Agric Food Chem. 2003;51:8067–8072. - PubMed
-
- Crispo JA, Piche M, Ansell DR, Eibl JK, Tai IT, Kumar A. Protective effects of methyl gallate on H2O2-induced apoptosis in PC12 cells. Biochem Biophys Res Commun. 2010;393:773–778. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

