Monoclonal antibodies against muscle actin isoforms: epitope identification and analysis of isoform expression by immunoblot and immunostaining in normal and regenerating skeletal muscle
- PMID: 27335638
- PMCID: PMC4893938
- DOI: 10.12688/f1000research.8154.2
Monoclonal antibodies against muscle actin isoforms: epitope identification and analysis of isoform expression by immunoblot and immunostaining in normal and regenerating skeletal muscle
Abstract
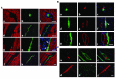
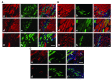
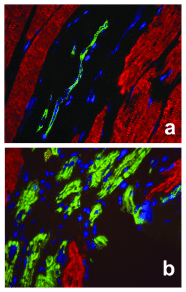
Higher vertebrates (mammals and birds) express six different highly conserved actin isoforms that can be classified in three subgroups: 1) sarcomeric actins, α-skeletal (α-SKA) and α-cardiac (α-CAA), 2) smooth muscle actins (SMAs), α-SMA and γ-SMA, and 3) cytoplasmic actins (CYAs), β-CYA and γ-CYA. The variations among isoactins, in each subgroup, are due to 3-4 amino acid differences located in their acetylated N-decapeptide sequence. The first monoclonal antibody (mAb) against an actin isoform (α-SMA) was produced and characterized in our laboratory in 1986 (Skalli et al., 1986) . We have further obtained mAbs against the 5 other isoforms. In this report, we focus on the mAbs anti-α-SKA and anti-α-CAA obtained after immunization of mice with the respective acetylated N-terminal decapeptides using the Repetitive Immunizations at Multiple Sites Strategy (RIMMS). In addition to the identification of their epitope by immunoblotting, we describe the expression of the 2 sarcomeric actins in mature skeletal muscle and during muscle repair after micro-lesions. In particular, we analyze the expression of α-CAA, α-SKA and α-SMA by co-immunostaining in a time course frame during the muscle repair process. Our results indicate that a restricted myocyte population expresses α-CAA and suggest a high capacity of self-regeneration in muscle cells. These antibodies may represent a helpful tool for the follow-up of muscle regeneration and pathological changes.
Keywords: Actin isoforms; epitope; monoclonal antibodies; muscle repair.
Conflict of interest statement
Figures


References
-
- Bochaton-Piallat M, Ropraz P, Gabbiani G, et al. : Actin isoform and intermediate filament protein expression in human developing skeletal muscle. Basic Appl Myol. 1992;2(2):83–87. Reference Source
-
- Chaponnier C, Gabbiani G: Dataset 1 in: Monoclonal antibodies against muscle actin isoforms: epitope identification and analysis of isoform expression by immunoblot and immunostaining in normal and regenerating skeletal muscle. F1000Research. 2016. Data Source - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

