Comparative kinematical analyses of Venus flytrap (Dionaea muscipula) snap traps
- PMID: 27335756
- PMCID: PMC4902084
- DOI: 10.3762/bjnano.7.59
Comparative kinematical analyses of Venus flytrap (Dionaea muscipula) snap traps
Abstract
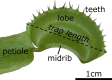
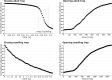
Although the Venus flytrap (Dionaea muscipula) can be considered as one of the most extensively investigated carnivorous plants, knowledge is still scarce about diversity of the snap-trap motion, the functionality of snap traps under varying environmental conditions, and their opening motion. By conducting simple snap-trap closure experiments in air and under water, we present striking evidence that adult Dionaea snaps similarly fast in aerial and submersed states and, hence, is potentially able to gain nutrients from fast aquatic prey during seasonal inundation. We reveal three snapping modes of adult traps, all incorporating snap buckling, and show that millimeter-sized, much slower seedling traps do not yet incorporate such elastic instabilities. Moreover, opening kinematics of young and adult Dionaea snap traps reveal that reverse snap buckling is not performed, corroborating the assumption that growth takes place on certain trap lobe regions. Our findings are discussed in an evolutionary, biomechanical, functional-morphological and biomimetic context.
Keywords: Droseraceae; biomechanics; carnivorous plant; fast plant movement; functional morphology.
Figures





References
-
- Darwin C. Insectivorous plants. London, United Kingdom: Murray; 1975. - DOI
-
- Juniper B E, Robins R J, Joel D M. The carnivorous plants. London, United Kingdom: Academic Press; 1989.
-
- Barthlott W, Porembski S, Seine R, et al. The curious world of carnivorous plants: a comprehensive guide to their biology and cultivation. Portland, U.S.A.: Timber; 2007.
LinkOut - more resources
Full Text Sources
Other Literature Sources
