Apatite precipitation on a novel fast-setting calcium silicate cement containing fluoride
- PMID: 27335901
- PMCID: PMC4894078
- DOI: 10.1080/23337931.2016.1178583
Apatite precipitation on a novel fast-setting calcium silicate cement containing fluoride
Abstract
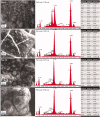
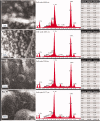
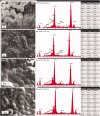
Aim: Calcium silicate cements are widely used in endodontics. Novel fast-setting calcium silicate cement with fluoride (Protooth) has been developed for potential applications in teeth crowns including cavity lining and cementation. Objective: To evaluate the surface apatite-forming ability of Protooth compositions as a function of fluoride content and immersion time in phosphate-buffered saline (PBS). Material and methods: Three cement compositions were tested: Protooth (3.5% fluoride and 10% radiocontrast), ultrafast Protooth (3.5% fluoride and 20% radiocontrast), and high fluoride Protooth (15% fluoride and 25% radiocontrast). Powders were cap-mixed with liquid, filled to the molds and immersed in PBS. Scanning electron microscopy, energy dispersive X-ray analysis, and Raman spectroscopy were used to characterize the precipitations morphology and composition after 1, 7, 28, and 56 days. Apatite/belite Raman peak height indicated the apatite thickness. Results: Spherical calcium phosphate precipitations with acicular crystallites were formed after 1-day immersion in PBS and Raman spectra disclosed the phosphate band at 965 cm-1, supporting the apatite formation over Protooth compositions. The apatite deposition continued and more voluminous precipitations were observed after 56 days over the surface of all cements. Raman bands suggested the formation of β-type carbonated apatite over Protooth compositions. High fluoride Protooth showed the most compact deposition with significantly higher apatite/belite ratio compared to Protooth and ultrafast Protooth after 28 and 56 days. Conclusions: Calcium phosphate precipitations (apatite) were formed over Protooth compositions after immersion in PBS with increasing apatite formation as a function of time. High fluoride Protooth exhibited thicker apatite deposition.
Keywords: Apatite; calcium silicate cement; fluoride; mineral trioxide aggregate.
Conflict of interest statement
Henrik Løvschall as patentee has financial relation with Dentosolve. The other authors declare that they have no conflict of interest.
Figures

Similar articles
-
Fluoride-containing nanoporous calcium-silicate MTA cements for endodontics and oral surgery: early fluorapatite formation in a phosphate-containing solution.Int Endod J. 2011 Oct;44(10):938-49. doi: 10.1111/j.1365-2591.2011.01907.x. Epub 2011 Jul 5. Int Endod J. 2011. PMID: 21726240
-
Environmental scanning electron microscopy connected with energy dispersive x-ray analysis and Raman techniques to study ProRoot mineral trioxide aggregate and calcium silicate cements in wet conditions and in real time.J Endod. 2010 May;36(5):851-7. doi: 10.1016/j.joen.2009.12.007. Epub 2010 Mar 4. J Endod. 2010. PMID: 20416432
-
Evaluation of the Ca ion release, pH and surface apatite formation of a prototype tricalcium silicate cement.Int Endod J. 2017 Dec;50 Suppl 2:e73-e82. doi: 10.1111/iej.12737. Epub 2017 Jan 16. Int Endod J. 2017. PMID: 27977862
-
Bond strength between dentine and a novel fast-setting calcium silicate cement with fluoride.Eur J Oral Sci. 2019 Dec;127(6):564-569. doi: 10.1111/eos.12659. Epub 2019 Dec 12. Eur J Oral Sci. 2019. PMID: 31830349
-
Calcium silicate bioactive cements: Biological perspectives and clinical applications.Dent Mater. 2015 Apr;31(4):351-70. doi: 10.1016/j.dental.2015.01.004. Epub 2015 Feb 7. Dent Mater. 2015. PMID: 25662204 Review.
Cited by
-
Delayed replantation of an avulsed immature permanent incisor and apexification using a novel fast-setting calcium silicate cement containing fluoride: a 3-year follow-up case report.Eur Arch Paediatr Dent. 2018 Apr;19(2):113-116. doi: 10.1007/s40368-017-0324-6. Epub 2018 Jan 12. Eur Arch Paediatr Dent. 2018. PMID: 29330841
-
Evaluating the shear bond strength and remineralization effect of calcium silicate-based and conventional self-adhesive resin cements to caries-affected dentin.Clin Exp Dent Res. 2022 Dec;8(6):1630-1637. doi: 10.1002/cre2.665. Epub 2022 Oct 3. Clin Exp Dent Res. 2022. PMID: 36189464 Free PMC article.
-
A Randomized Split Mouth Clinical Trial Comparing Mineral Trioxide Aggregate with a New Fast-setting Calcium Silicate Cement in Direct Pulp Capping of Primary Molars: A Preliminary Report from a Long-term Follow-up.Int J Clin Pediatr Dent. 2020 Jul-Aug;13(4):390-394. doi: 10.5005/jp-journals-10005-1801. Int J Clin Pediatr Dent. 2020. Retraction in: Int J Clin Pediatr Dent. 2020 Jul-Aug;13(4):838. doi: 10.5005/jp-journals-10005-2177. PMID: 33149412 Free PMC article. Retracted.
-
Pull-Out Bond Strength of Titanium Post Cemented with Novel Fast-Setting Calcium Silicate Cement.Eur Endod J. 2021 Dec;6(3):314-318. doi: 10.14744/eej.2021.69875. Eur Endod J. 2021. PMID: 34967340 Free PMC article.
-
Cytocompatibility and cell proliferation evaluation of calcium phosphate-based root canal sealers.Restor Dent Endod. 2019 Nov 15;45(1):e2. doi: 10.5395/rde.2020.45.e2. eCollection 2020 Feb. Restor Dent Endod. 2019. PMID: 32110532 Free PMC article.
References
-
- Reyes-Carmona JF, Felippe MS, Felippe WT.. Biomineralization ability and interaction of mineral trioxide aggregate and white Portland cement with dentin in a phosphate-containing fluid. J Endod. 2009;35:731–736. - PubMed
-
- Sarkar NK, Caicedo R, Ritwik P, et al. . Physicochemical basis of the biologic properties of mineral trioxide aggregate. J Endod. 2005;31:97–100. - PubMed
-
- Martin RL, Monticelli F, Brackett WW, et al. . Sealing properties of mineral trioxide aggregate orthograde apical plugs and root fillings in an in vitro apexification model. J Endod. 2007;33:272–275. - PubMed
-
- Eldeniz AU, Hadimli HH, Ataoglu H, et al. . Antibacterial effect of selected root-end filling materials. J Endod. 2006;32:345–349. - PubMed
-
- Kogan P, He J, Glickman GN, et al. . The effects of various additives on setting properties of MTA. J Endod. 2006;32:569–572. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
