Multi-epitope Models Explain How Pre-existing Antibodies Affect the Generation of Broadly Protective Responses to Influenza
- PMID: 27336297
- PMCID: PMC4918916
- DOI: 10.1371/journal.ppat.1005692
Multi-epitope Models Explain How Pre-existing Antibodies Affect the Generation of Broadly Protective Responses to Influenza
Abstract
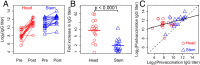
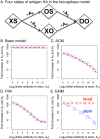
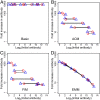
The development of next-generation influenza vaccines that elicit strain-transcendent immunity against both seasonal and pandemic viruses is a key public health goal. Targeting the evolutionarily conserved epitopes on the stem of influenza's major surface molecule, hemagglutinin, is an appealing prospect, and novel vaccine formulations show promising results in animal model systems. However, studies in humans indicate that natural infection and vaccination result in limited boosting of antibodies to the stem of HA, and the level of stem-specific antibody elicited is insufficient to provide broad strain-transcendent immunity. Here, we use mathematical models of the humoral immune response to explore how pre-existing immunity affects the ability of vaccines to boost antibodies to the head and stem of HA in humans, and, in particular, how it leads to the apparent lack of boosting of broadly cross-reactive antibodies to the stem epitopes. We consider hypotheses where binding of antibody to an epitope: (i) results in more rapid clearance of the antigen; (ii) leads to the formation of antigen-antibody complexes which inhibit B cell activation through Fcγ receptor-mediated mechanism; and (iii) masks the epitope and prevents the stimulation and proliferation of specific B cells. We find that only epitope masking but not the former two mechanisms to be key in recapitulating patterns in data. We discuss the ramifications of our findings for the development of vaccines against both seasonal and pandemic influenza.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Molinari NAM, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007. June;25(27):5086–96. - PubMed
-
- Yewdell J, García-Sastre A. Influenza virus still surprises. Curr Opin Microbiol. 2002. August;5(4):414–8. - PubMed
-
- Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012. January;12(1):36–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

