Electrophysiology of Heart Failure Using a Rabbit Model: From the Failing Myocyte to Ventricular Fibrillation
- PMID: 27336310
- PMCID: PMC4919062
- DOI: 10.1371/journal.pcbi.1004968
Electrophysiology of Heart Failure Using a Rabbit Model: From the Failing Myocyte to Ventricular Fibrillation
Abstract
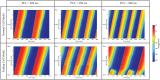
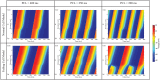
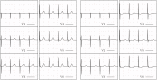
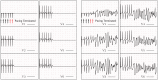
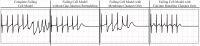
Heart failure is a leading cause of death, yet its underlying electrophysiological (EP) mechanisms are not well understood. In this study, we use a multiscale approach to analyze a model of heart failure and connect its results to features of the electrocardiogram (ECG). The heart failure model is derived by modifying a previously validated electrophysiology model for a healthy rabbit heart. Specifically, in accordance with the heart failure literature, we modified the cell EP by changing both membrane currents and calcium handling. At the tissue level, we modeled the increased gap junction lateralization and lower conduction velocity due to downregulation of Connexin 43. At the biventricular level, we reduced the apex-to-base and transmural gradients of action potential duration (APD). The failing cell model was first validated by reproducing the longer action potential, slower and lower calcium transient, and earlier alternans characteristic of heart failure EP. Subsequently, we compared the electrical wave propagation in one dimensional cables of healthy and failing cells. The validated cell model was then used to simulate the EP of heart failure in an anatomically accurate biventricular rabbit model. As pacing cycle length decreases, both the normal and failing heart develop T-wave alternans, but only the failing heart shows QRS alternans (although moderate) at rapid pacing. Moreover, T-wave alternans is significantly more pronounced in the failing heart. At rapid pacing, APD maps show areas of conduction block in the failing heart. Finally, accelerated pacing initiated wave reentry and breakup in the failing heart. Further, the onset of VF was not observed with an upregulation of SERCA, a potential drug therapy, using the same protocol. The changes introduced at the cell and tissue level have increased the failing heart's susceptibility to dynamic instabilities and arrhythmias under rapid pacing. However, the observed increase in arrhythmogenic potential is not due to a steepening of the restitution curve (not present in our model), but rather to a novel blocking mechanism.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

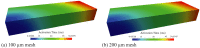






Similar articles
-
Mechanisms linking electrical alternans and clinical ventricular arrhythmia in human heart failure.Heart Rhythm. 2016 Sep;13(9):1922-31. doi: 10.1016/j.hrthm.2016.05.017. Epub 2016 May 20. Heart Rhythm. 2016. PMID: 27215536 Free PMC article.
-
Piceatannol facilitates conduction block and ventricular fibrillation induction in ischemia-reperfused rabbit hearts with pacing-induced heart failure.Int J Cardiol. 2014 Feb 1;171(2):250-8. doi: 10.1016/j.ijcard.2013.12.033. Epub 2013 Dec 22. Int J Cardiol. 2014. PMID: 24388545
-
Developing a novel comprehensive framework for the investigation of cellular and whole heart electrophysiology in the in situ human heart: historical perspectives, current progress and future prospects.Prog Biophys Mol Biol. 2014 Aug;115(2-3):252-60. doi: 10.1016/j.pbiomolbio.2014.06.004. Epub 2014 Jun 24. Prog Biophys Mol Biol. 2014. PMID: 24972083 Review.
-
Heart failure enhances susceptibility to arrhythmogenic cardiac alternans.Heart Rhythm. 2009 Feb;6(2):251-9. doi: 10.1016/j.hrthm.2008.11.008. Epub 2008 Nov 8. Heart Rhythm. 2009. PMID: 19187920 Free PMC article.
-
Arrhythmia mechanisms in the failing heart.Pacing Clin Electrophysiol. 2008 Aug;31(8):1048-56. doi: 10.1111/j.1540-8159.2008.01134.x. Pacing Clin Electrophysiol. 2008. PMID: 18684263 Review.
Cited by
-
High-Resolution Ex Vivo Microstructural MRI After Restoring Ventricular Geometry via 3D Printing.Funct Imaging Model Heart. 2019 Jun;11504:177-186. doi: 10.1007/978-3-030-21949-9_20. Epub 2019 May 30. Funct Imaging Model Heart. 2019. PMID: 31432042 Free PMC article.
-
Effects of pharmacological gap junction and sodium channel blockade on S1S2 restitution properties in Langendorff-perfused mouse hearts.Oncotarget. 2017 Jul 28;8(49):85341-85352. doi: 10.18632/oncotarget.19675. eCollection 2017 Oct 17. Oncotarget. 2017. PMID: 29156723 Free PMC article.
-
Mechanistic investigation of Ca2+ alternans in human heart failure and its modulation by fibroblasts.PLoS One. 2019 Jun 18;14(6):e0217993. doi: 10.1371/journal.pone.0217993. eCollection 2019. PLoS One. 2019. PMID: 31211790 Free PMC article.
-
Semi-implicit Non-conforming Finite-Element Schemes for Cardiac Electrophysiology: A Framework for Mesh-Coarsening Heart Simulations.Front Physiol. 2018 Oct 30;9:1513. doi: 10.3389/fphys.2018.01513. eCollection 2018. Front Physiol. 2018. PMID: 30425648 Free PMC article.
-
Microstructural Infarct Border Zone Remodeling in the Post-infarct Swine Heart Measured by Diffusion Tensor MRI.Front Physiol. 2018 Aug 22;9:826. doi: 10.3389/fphys.2018.00826. eCollection 2018. Front Physiol. 2018. PMID: 30246802 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

