Mitofusin-mediated ER stress triggers neurodegeneration in pink1/parkin models of Parkinson's disease
- PMID: 27336715
- PMCID: PMC5143399
- DOI: 10.1038/cddis.2016.173
Mitofusin-mediated ER stress triggers neurodegeneration in pink1/parkin models of Parkinson's disease
Abstract
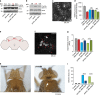
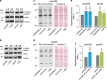
Mutations in PINK1 and PARKIN cause early-onset Parkinson's disease (PD), thought to be due to mitochondrial toxicity. Here, we show that in Drosophila pink1 and parkin mutants, defective mitochondria also give rise to endoplasmic reticulum (ER) stress signalling, specifically to the activation of the protein kinase R-like endoplasmic reticulum kinase (PERK) branch of the unfolded protein response (UPR). We show that enhanced ER stress signalling in pink1 and parkin mutants is mediated by mitofusin bridges, which occur between defective mitochondria and the ER. Reducing mitofusin contacts with the ER is neuroprotective, through suppression of PERK signalling, while mitochondrial dysfunction remains unchanged. Further, both genetic inhibition of dPerk-dependent ER stress signalling and pharmacological inhibition using the PERK inhibitor GSK2606414 were neuroprotective in both pink1 and parkin mutants. We conclude that activation of ER stress by defective mitochondria is neurotoxic in pink1 and parkin flies and that the reduction of this signalling is neuroprotective, independently of defective mitochondria. A video abstract for this article is available online in the supplementary information.
Figures



References
-
- Halliday M, Mallucci GR. Targeting the unfolded protein response in neurodegeneration: a new approach to therapy. Neuropharmacology 2014; 76 Pt A: 169–174. - PubMed
-
- Moreno JA, Halliday M, Molloy C, Radford H, Verity N, Axten JM et al. Oral treatment targeting the unfolded protein response prevents neurodegeneration and clinical disease in prion-infected mice. Sci Transl Med 2013; 5: 206ra138. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

