STAT3 modulates β-cell cycling in injured mouse pancreas and protects against DNA damage
- PMID: 27336716
- PMCID: PMC5143397
- DOI: 10.1038/cddis.2016.171
STAT3 modulates β-cell cycling in injured mouse pancreas and protects against DNA damage
Abstract
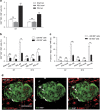
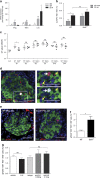
Partial pancreatic duct ligation (PDL) of mouse pancreas induces a doubling of the β-cell mass mainly through proliferation of pre-existing and newly formed β-cells. The molecular mechanism governing this process is still largely unknown. Given the inflammatory nature of PDL and inflammation-induced signaling via the signal transducer and activator of transcription 3 (STAT3), the activation and the role of STAT3 in PDL-induced β-cell proliferation were investigated. Duct ligation stimulates the expression of several cytokines that can act as ligands inducing STAT3 signaling and phosphorylation in β-cells. β-Cell cycling increased by conditional β-cell-specific Stat3 knockout and decreased by STAT3 activation through administration of interleukin-6. In addition, the level of DNA damage in β-cells of PDL pancreas increased after deletion of Stat3. These data indicate a role for STAT3 in maintaining a steady state in the β-cell, by modulating its cell cycle and protection from DNA damage.
Figures



Similar articles
-
Prevention of Reg I-induced β-cell apoptosis by IL-6/dexamethasone through activation of HGF gene regulation.Biochim Biophys Acta. 2013 Dec;1833(12):2988-2995. doi: 10.1016/j.bbamcr.2013.08.004. Epub 2013 Aug 14. Biochim Biophys Acta. 2013. PMID: 23954444
-
Surgical Injury to the Mouse Pancreas through Ligation of the Pancreatic Duct as a Model for Endocrine and Exocrine Reprogramming and Proliferation.J Vis Exp. 2015 Aug 7;(102):e52765. doi: 10.3791/52765. J Vis Exp. 2015. PMID: 26273954 Free PMC article.
-
SMAD3/Stat3 Signaling Mediates β-Cell Epithelial-Mesenchymal Transition in Chronic Pancreatitis-Related Diabetes.Diabetes. 2017 Oct;66(10):2646-2658. doi: 10.2337/db17-0537. Epub 2017 Aug 3. Diabetes. 2017. PMID: 28775125 Free PMC article.
-
β-Cells are not generated in pancreatic duct ligation-induced injury in adult mice.Diabetes. 2013 May;62(5):1634-45. doi: 10.2337/db12-0848. Epub 2013 Jan 24. Diabetes. 2013. PMID: 23349489 Free PMC article.
-
Suppression of SOCS3 expression in the pancreatic beta-cell leads to resistance to type 1 diabetes.Biochem Biophys Res Commun. 2007 Aug 10;359(4):952-8. doi: 10.1016/j.bbrc.2007.05.198. Epub 2007 Jun 4. Biochem Biophys Res Commun. 2007. PMID: 17562326
Cited by
-
Understanding cell fate acquisition in stem-cell-derived pancreatic islets using single-cell multiome-inferred regulomes.Dev Cell. 2023 May 8;58(9):727-743.e11. doi: 10.1016/j.devcel.2023.03.011. Epub 2023 Apr 10. Dev Cell. 2023. PMID: 37040771 Free PMC article.
-
Inhibition of the STAT3/Fanconi anemia axis is synthetic lethal with PARP inhibition in breast cancer.Nat Commun. 2025 Mar 4;16(1):2159. doi: 10.1038/s41467-025-57476-4. Nat Commun. 2025. PMID: 40038300 Free PMC article.
-
Loss of Human Beta Cell Identity in a Reconstructed Omental Stromal Cell Environment.Cells. 2022 Mar 8;11(6):924. doi: 10.3390/cells11060924. Cells. 2022. PMID: 35326375 Free PMC article.
-
Endoluminal radiofrequency ablation of the main pancreatic duct is a secure and effective method to produce pancreatic atrophy and to achieve stump closure.Sci Rep. 2019 Apr 11;9(1):5928. doi: 10.1038/s41598-019-42411-7. Sci Rep. 2019. PMID: 30976043 Free PMC article.
-
HJC0152 suppresses human non-small-cell lung cancer by inhibiting STAT3 and modulating metabolism.Cell Prolif. 2020 Mar;53(3):e12777. doi: 10.1111/cpr.12777. Epub 2020 Feb 5. Cell Prolif. 2020. PMID: 32022328 Free PMC article.
References
-
- Levy DE, Darnell JE Jr. Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol 2002; 3: 651–662. - PubMed
-
- Kisseleva T, Bhattacharya S, Braunstein J, Schindler CW. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene 2002; 285: 1–24. - PubMed
-
- Kira M, Sano S, Takagi S, Yoshikawa K, Takeda J, Itami S. STAT3 deficiency in keratinocytes leads to compromised cell migration through hyperphosphorylation of p130(cas). J Biol Chem 2002; 277: 12931–12936. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

