Junctophilin 3 expresses in pancreatic beta cells and is required for glucose-stimulated insulin secretion
- PMID: 27336719
- PMCID: PMC5143404
- DOI: 10.1038/cddis.2016.179
Junctophilin 3 expresses in pancreatic beta cells and is required for glucose-stimulated insulin secretion
Abstract
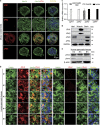
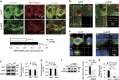
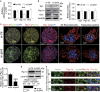
It is well accepted that junctophilin (JPHs) isoforms act as a physical bridge linking plasma membrane and endoplasmic reticulum (ER) for channel crosstalk in excitable cells. Our purpose is to investigate whether JPHs are involved in the proper communication between Ca(2+) influx and subsequent Ca(2+) amplification in pancreatic beta cells, thereby participating in regulating insulin secretion. The expression of JPH isoforms was examined in human and mouse pancreatic tissues, and JPH3 expression was found in both the beta cells. In mice, knockdown of Jph3 (si-Jph3) in islets decreased glucose-stimulated insulin secretion (GSIS) accompanied by mitochondrial function impairment. Si-Jph3 lowered the insulin secretory response to Ca(2+) signaling in the presence of glucose, and reduced [Ca(2+)]c transient amplitude triggered by caffeine. Si-Jph3 also attenuated mitofusin 2 expression, thereby disturbing the spatial organization of ER-mitochondria contact in islets. These results suggest that the regulation of GSIS by the KATP channel-independent pathways is partly impaired due to decrease of JPH3 expression in mouse islets. JPH3 also binds to type 2 ryanodine receptors (RyR2) in mouse and human pancreatic tissues, which might contribute to Ca(2+) release amplification in GSIS. This study demonstrates some previously unrecognized findings in pancreatic tissues: (1) JPH3 expresses in mouse and human beta cells; (2) si-Jph3 in mouse primary islets impairs GSIS in vitro; (3) impairment in GSIS in si-Jph3 islets is due to changes in RyR2-[Ca(2+)]c transient amplitude and ER-mitochondria contact.
Figures



References
-
- Ravier MA, Daro D, Roma LP, Jonas JC, Cheng-Xue R, Schuit FC et al. Mechanisms of control of the free Ca2+ concentration in the endoplasmic reticulum of mouse pancreatic beta-cells: interplay with cell metabolism and [Ca2+]c and role of SERCA2b and SERCA3. Diabetes 2011; 60: 2533–2545. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

