In Vitro Infection with Dengue Virus Induces Changes in the Structure and Function of the Mouse Brain Endothelium
- PMID: 27336851
- PMCID: PMC4919088
- DOI: 10.1371/journal.pone.0157786
In Vitro Infection with Dengue Virus Induces Changes in the Structure and Function of the Mouse Brain Endothelium
Abstract
Background: The neurological manifestations of dengue disease are occurring with greater frequency, and currently, no information is available regarding the reasons for this phenomenon. Some viruses infect and/or alter the function of endothelial organs, which results in changes in cellular function, including permeability of the blood-brain barrier (BBB), which allows the entry of infected cells or free viral particles into the nervous system.
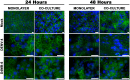
Methods: In the present study, we standardized two in vitro models, a polarized monolayer of mouse brain endothelial cells (MBECs) and an organized co-culture containing MBECs and astrocytes. Using these cell models, we assessed whether DENV-4 or the neuro-adapted dengue virus (D4MB-6) variant infects cells or induces changes in the structure or function of the endothelial barrier.
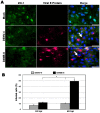
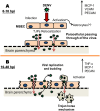
Results: The results showed that MBECs, but not astrocytes, were susceptible to infection with both viruses, although the percentage of infected cells was higher when the neuro-adapted virus variant was used. In both culture systems, DENV infection changed the localization of the tight junction proteins Zonula occludens (ZO-1) and Claudin-1 (Cln1), and this process was associated with a decrease in transendothelial resistance, an increase in macromolecule permeability and an increase in the paracellular passing of free virus particles. MBEC infection led to transcriptional up-regulation of adhesion molecules (VCAM-1 and PECAM) and immune mediators (MCP-1 and TNF- α) that are associated with immune cell transmigration, mainly in D4MB-6-infected cells.
Conclusion: These results indicate that DENV infection in MBECs altered the structure and function of the BBB and activated the endothelium, affecting its transcellular and paracellular permeability and favoring the passage of viruses and the transmigration of immune cells. This phenomenon can be harnessed for neurotropic and neurovirulent strains to infect and induce alterations in the CNS.
Conflict of interest statement
Figures




Similar articles
-
Invasion of a murine in vitro blood-brain barrier co-culture model by dengue virus serotypes 1 to 4.Arch Virol. 2019 Apr;164(4):1069-1083. doi: 10.1007/s00705-019-04175-3. Epub 2019 Feb 19. Arch Virol. 2019. PMID: 30783772
-
High dengue virus load differentially modulates human microvascular endothelial barrier function during early infection.J Gen Virol. 2017 Dec;98(12):2993-3007. doi: 10.1099/jgv.0.000981. Epub 2017 Nov 24. J Gen Virol. 2017. PMID: 29182510
-
West Nile virus-induced cell adhesion molecules on human brain microvascular endothelial cells regulate leukocyte adhesion and modulate permeability of the in vitro blood-brain barrier model.PLoS One. 2014 Jul 18;9(7):e102598. doi: 10.1371/journal.pone.0102598. eCollection 2014. PLoS One. 2014. PMID: 25036379 Free PMC article.
-
Transcytosis Involvement in Transport System and Endothelial Permeability of Vascular Leakage during Dengue Virus Infection.Viruses. 2018 Feb 8;10(2):69. doi: 10.3390/v10020069. Viruses. 2018. PMID: 29419739 Free PMC article. Review.
-
Endothelial dysfunction in dengue virus pathology.Rev Med Virol. 2015 Jan;25(1):50-67. doi: 10.1002/rmv.1818. Epub 2014 Nov 27. Rev Med Virol. 2015. PMID: 25430853 Review.
Cited by
-
Endothelial Dysfunction, HMGB1, and Dengue: An Enigma to Solve.Viruses. 2022 Aug 12;14(8):1765. doi: 10.3390/v14081765. Viruses. 2022. PMID: 36016387 Free PMC article. Review.
-
Neurocognitive impacts of arbovirus infections.J Neuroinflammation. 2020 Aug 10;17(1):233. doi: 10.1186/s12974-020-01904-3. J Neuroinflammation. 2020. PMID: 32778106 Free PMC article. Review.
-
Case Report: Right Hemispheric Neuroimaging Abnormalities in a Patient with Dengue Encephalopathy.Am J Trop Med Hyg. 2018 Nov;99(5):1291-1293. doi: 10.4269/ajtmh.18-0431. Am J Trop Med Hyg. 2018. PMID: 30334519 Free PMC article.
-
Mechanisms of Neuroinvasion and Neuropathogenesis by Pathologic Flaviviruses.Viruses. 2023 Jan 17;15(2):261. doi: 10.3390/v15020261. Viruses. 2023. PMID: 36851477 Free PMC article. Review.
-
Pathways Exploited by Flaviviruses to Counteract the Blood-Brain Barrier and Invade the Central Nervous System.Front Microbiol. 2019 Mar 28;10:525. doi: 10.3389/fmicb.2019.00525. eCollection 2019. Front Microbiol. 2019. PMID: 30984122 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

