Autophagy deficiency in myeloid cells increases susceptibility to obesity-induced diabetes and experimental colitis
- PMID: 27337687
- PMCID: PMC4968230
- DOI: 10.1080/15548627.2016.1184799
Autophagy deficiency in myeloid cells increases susceptibility to obesity-induced diabetes and experimental colitis
Abstract
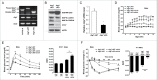
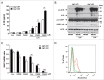
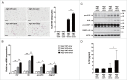
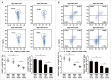
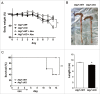
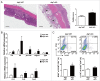
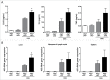
Autophagy, which is critical for the proper turnover of organelles such as endoplasmic reticulum and mitochondria, affects diverse aspects of metabolism, and its dysregulation has been incriminated in various metabolic disorders. However, the role of autophagy of myeloid cells in adipose tissue inflammation and type 2 diabetes has not been addressed. We produced mice with myeloid cell-specific deletion of Atg7 (autophagy-related 7), an essential autophagy gene (Atg7 conditional knockout [cKO] mice). While Atg7 cKO mice were metabolically indistinguishable from control mice, they developed diabetes when bred to ob/w mice (Atg7 cKO-ob/ob mice), accompanied by increases in the crown-like structure, inflammatory cytokine expression and inflammasome activation in adipose tissue. Mφs (macrophages) from Atg7 cKO mice showed significantly higher interleukin 1 β release and inflammasome activation in response to a palmitic acid plus lipopolysaccharide combination. Moreover, a decrease in the NAD(+):NADH ratio and increase in intracellular ROS content after treatment with palmitic acid in combination with lipopolysaccharide were more pronounced in Mφs from Atg7 cKO mice, suggesting that mitochondrial dysfunction in autophagy-deficient Mφs leads to an increase in lipid-induced inflammasome and metabolic deterioration in Atg7 cKO-ob/ob mice. Atg7 cKO mice were more susceptible to experimental colitis, accompanied by increased colonic cytokine expression, T helper 1 skewing and systemic bacterial invasion. These results suggest that autophagy of Mφs is important for the control of inflammasome activation in response to metabolic or extrinsic stress, and autophagy deficiency in Mφs may contribute to the progression of metabolic syndrome associated with lipid injury and colitis.
Keywords: autophagy; colitis; diabetes; inflammasome; macrophages; obesity.
Figures
Similar articles
-
Systemic autophagy insufficiency compromises adaptation to metabolic stress and facilitates progression from obesity to diabetes.Nat Commun. 2014 Sep 26;5:4934. doi: 10.1038/ncomms5934. Nat Commun. 2014. PMID: 25255859
-
Role of autophagy in the progression from obesity to diabetes and in the control of energy balance.Arch Pharm Res. 2013 Feb;36(2):223-9. doi: 10.1007/s12272-013-0024-7. Epub 2013 Jan 31. Arch Pharm Res. 2013. PMID: 23371805 Review.
-
Inflammasome, mTORC1 activation, and metabolic derangement contribute to the susceptibility of diabetics to infections.Med Hypotheses. 2015 Dec;85(6):997-1001. doi: 10.1016/j.mehy.2015.08.019. Epub 2015 Sep 6. Med Hypotheses. 2015. PMID: 26384528
-
Autophagy deficiency in beta cells leads to compromised unfolded protein response and progression from obesity to diabetes in mice.Diabetologia. 2012 Feb;55(2):392-403. doi: 10.1007/s00125-011-2350-y. Epub 2011 Nov 11. Diabetologia. 2012. PMID: 22075916
-
Role of autophagy in diabetes and endoplasmic reticulum stress of pancreatic β-cells.Exp Mol Med. 2012 Feb 29;44(2):81-8. doi: 10.3858/emm.2012.44.2.030. Exp Mol Med. 2012. PMID: 22257883 Free PMC article. Review.
Cited by
-
Identification of Pharmacological Autophagy Regulators of Active Ulcerative Colitis.Front Pharmacol. 2021 Dec 1;12:769718. doi: 10.3389/fphar.2021.769718. eCollection 2021. Front Pharmacol. 2021. PMID: 34925026 Free PMC article.
-
The Role of Autophagy in Eosinophilic Airway Inflammation.Immune Netw. 2019 Feb 4;19(1):e5. doi: 10.4110/in.2019.19.e5. eCollection 2019 Feb. Immune Netw. 2019. PMID: 30838160 Free PMC article. Review.
-
Beclin 1 functions as a negative modulator of MLKL oligomerisation by integrating into the necrosome complex.Cell Death Differ. 2020 Nov;27(11):3065-3081. doi: 10.1038/s41418-020-0561-9. Epub 2020 May 26. Cell Death Differ. 2020. PMID: 32457484 Free PMC article.
-
Myeloid ATG16L1 does not affect adipose tissue inflammation or body mass in mice fed high fat diet.Obes Res Clin Pract. 2018 Mar-Apr;12(2):174-186. doi: 10.1016/j.orcp.2017.10.006. Epub 2017 Nov 2. Obes Res Clin Pract. 2018. PMID: 29103907 Free PMC article.
-
ESRRA (estrogen related receptor alpha) is a critical regulator of intestinal homeostasis through activation of autophagic flux via gut microbiota.Autophagy. 2021 Oct;17(10):2856-2875. doi: 10.1080/15548627.2020.1847460. Epub 2020 Dec 15. Autophagy. 2021. PMID: 33172329 Free PMC article.
References
-
- Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell 2011; 147:728-41; PMID:22078875; http://dx.doi.org/10.1016/j.cell.2011.10.026 - DOI - PubMed
-
- Ozcan U, Cao Q, Yilmaz E, Lee A-H, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 Diabetes. Science 2004; 306:457-61; PMID:15486293; http://dx.doi.org/10.1126/science.1103160 - DOI - PubMed
-
- Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 2003; 300:1140-2; PMID:12750520; http://dx.doi.org/10.1126/science.1082889 - DOI - PMC - PubMed
-
- Quan W, Hur KY, Lim Y, Oh SH, Lee J-C, Kim HC, Kim G-H, Kim S-H, Kim HL, Lee M-K, et al.. Autophagy deficiency in beta cells leads to compromised unfolded protein response and progression from obesity to diabetes in mice. Diabetologia 2012; 55:392-403; PMID:22075916; http://dx.doi.org/10.1007/s00125-011-2350-y - DOI - PubMed
-
- Kim KH, Lee M-S. Autophagy-a key player in cellular and body metabolism. Nat Rev Endocrinol 2014; 10:322-37; PMID:24663220; http://dx.doi.org/10.1038/nrendo.2014.35 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
