Polyphosphate is a novel cofactor for regulation of complement by a serpin, C1 inhibitor
- PMID: 27338096
- PMCID: PMC5043130
- DOI: 10.1182/blood-2016-02-699561
Polyphosphate is a novel cofactor for regulation of complement by a serpin, C1 inhibitor
Abstract
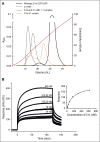
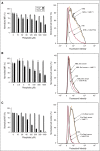
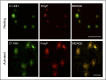
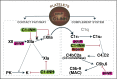
The complement system plays a key role in innate immunity, inflammation, and coagulation. The system is delicately balanced by negative regulatory mechanisms that modulate the host response to pathogen invasion and injury. The serpin, C1-esterase inhibitor (C1-INH), is the only known plasma inhibitor of C1s, the initiating serine protease of the classical pathway of complement. Like other serpin-protease partners, C1-INH interaction with C1s is accelerated by polyanions such as heparin. Polyphosphate (polyP) is a naturally occurring polyanion with effects on coagulation and complement. We recently found that polyP binds to C1-INH, prompting us to consider whether polyP acts as a cofactor for C1-INH interactions with its target proteases. We show that polyP dampens C1s-mediated activation of the classical pathway in a polymer length- and concentration-dependent manner by accelerating C1-INH neutralization of C1s cleavage of C4 and C2. PolyP significantly increases the rate of interaction between C1s and C1-INH, to an extent comparable to heparin, with an exosite on the serine protease domain of the enzyme playing a major role in this interaction. In a serum-based cell culture system, polyP significantly suppressed C4d deposition on endothelial cells, generated via the classical and lectin pathways. Moreover, polyP and C1-INH colocalize in activated platelets, suggesting that their interactions are physiologically relevant. In summary, like heparin, polyP is a naturally occurring cofactor for the C1s:C1-INH interaction and thus an important regulator of complement activation. The findings may provide novel insights into mechanisms underlying inflammatory diseases and the development of new therapies.
© 2016 by The American Society of Hematology.
Figures



Comment in
-
PolyP's many faces.Blood. 2016 Sep 29;128(13):1669-70. doi: 10.1182/blood-2016-07-724971. Blood. 2016. PMID: 27688781 Free PMC article.
References
-
- Ziccardi RJ. Activation of the early components of the classical complement pathway under physiologic conditions. J Immunol. 1981;126(5):1769–1773. - PubMed
-
- Rossi V, Cseh S, Bally I, Thielens NM, Jensenius JC, Arlaud GJ. Substrate specificities of recombinant mannan-binding lectin-associated serine proteases-1 and -2. J Biol Chem. 2001;276(44):40880–40887. - PubMed
-
- Wuillemin WA, Eldering E, Citarella F, de Ruig CP, ten Cate H, Hack CE. Modulation of contact system proteases by glycosaminoglycans. Selective enhancement of the inhibition of factor XIa. J Biol Chem. 1996;271(22):12913–12918. - PubMed
-
- Wuillemin WA, Minnema M, Meijers JC, et al. Inactivation of factor XIa in human plasma assessed by measuring factor XIa-protease inhibitor complexes: major role for C1-inhibitor. Blood. 1995;85(6):1517–1526. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

