Development of an in Silico Model of DPPH• Free Radical Scavenging Capacity: Prediction of Antioxidant Activity of Coumarin Type Compounds
- PMID: 27338348
- PMCID: PMC4926415
- DOI: 10.3390/ijms17060881
Development of an in Silico Model of DPPH• Free Radical Scavenging Capacity: Prediction of Antioxidant Activity of Coumarin Type Compounds
Abstract
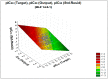
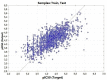
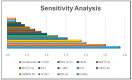
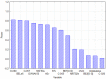
A quantitative structure-activity relationship (QSAR) study of the 2,2-diphenyl-l-picrylhydrazyl (DPPH•) radical scavenging ability of 1373 chemical compounds, using DRAGON molecular descriptors (MD) and the neural network technique, a technique based on the multilayer multilayer perceptron (MLP), was developed. The built model demonstrated a satisfactory performance for the training ( R 2 = 0.713 ) and test set ( Q ext 2 = 0.654 ) , respectively. To gain greater insight on the relevance of the MD contained in the MLP model, sensitivity and principal component analyses were performed. Moreover, structural and mechanistic interpretation was carried out to comprehend the relationship of the variables in the model with the modeled property. The constructed MLP model was employed to predict the radical scavenging ability for a group of coumarin-type compounds. Finally, in order to validate the model's predictions, an in vitro assay for one of the compounds (4-hydroxycoumarin) was performed, showing a satisfactory proximity between the experimental and predicted pIC50 values.
Keywords: DPPH•; MLP; QSAR; antioxidant; artificial neural networks; coumarin; free radical scavenger.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

