Expression of Stipa purpurea SpCIPK26 in Arabidopsis thaliana Enhances Salt and Drought Tolerance and Regulates Abscisic Acid Signaling
- PMID: 27338368
- PMCID: PMC4926498
- DOI: 10.3390/ijms17060966
Expression of Stipa purpurea SpCIPK26 in Arabidopsis thaliana Enhances Salt and Drought Tolerance and Regulates Abscisic Acid Signaling
Abstract
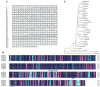
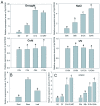
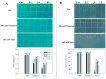
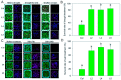
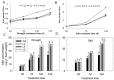
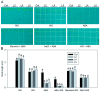
Stipa purpurea (S. purpurea) is the dominant plant species in the alpine steppe of the Qinghai-Tibet Plateau, China. It is highly resistant to cold and drought conditions. However, the underlying mechanisms regulating the stress tolerance are unknown. In this study, a CIPK gene from S. purpurea (SpCIPK26) was isolated. The SpCIPK26 coding region consisted of 1392 bp that encoded 464 amino acids. The protein has a highly conserved catalytic structure and regulatory domain. The expression of SpCIPK26 was induced by drought and salt stress. SpCIPK26 overexpression in Arabidopsis thaliana (A. thaliana) plants provided increased tolerance to drought and salt stress in an abscisic acid (ABA)-dependent manner. Compared with wild-type A. thaliana plants, SpCIPK26-overexpressing plants had higher survival rates, water potentials, and photosynthetic efficiency (Fv/Fm), as well as lower levels of reactive oxygen species (ROS) following exposure to drought and salt stress. Gene expression analyses indicated stress-inducible genes (RD29A, RD29B, and ABF2) and a ROS-scavenger gene (CAT1) were upregulated in SpCIPK26-overexpressing plants after stress treatments. All of these marker genes are associated with ABA-responsive cis-acting elements. Additionally, the similarities in the gene expression patterns following ABA, mannitol, and NaCl treatments suggest SpCIPK26 has an important role during plant responses to drought and salt stress and in regulating ABA signaling.
Keywords: ABA; CIPK; ROS; Stipa purpurea; drought; salt.
Figures




References
-
- Ibraheem O., Dealtry G., Roux S., Bradley G. The effect of drought and salinity on the expressional levels of sucrose transporters in rice (Oryza sativa Nipponbare) cultivar plants. Plant Omics. 2011;4:68–74.
-
- Llanes A., Andrade A., Masciarelli O., Alemano S., Luna V. Drought and salinity alter endogenous hormonal profiles at the seed germination phase. Seed Sci. Res. 2016;26:1–13. doi: 10.1017/S0960258515000331. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

