Endoplasmic Reticulum: The Favorite Intracellular Niche for Viral Replication and Assembly
- PMID: 27338443
- PMCID: PMC4926180
- DOI: 10.3390/v8060160
Endoplasmic Reticulum: The Favorite Intracellular Niche for Viral Replication and Assembly
Abstract
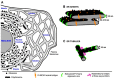
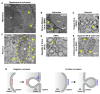
The endoplasmic reticulum (ER) is the largest intracellular organelle. It forms a complex network of continuous sheets and tubules, extending from the nuclear envelope (NE) to the plasma membrane. This network is frequently perturbed by positive-strand RNA viruses utilizing the ER to create membranous replication factories (RFs), where amplification of their genomes occurs. In addition, many enveloped viruses assemble progeny virions in association with ER membranes, and viruses replicating in the nucleus need to overcome the NE barrier, requiring transient changes of the NE morphology. This review first summarizes some key aspects of ER morphology and then focuses on the exploitation of the ER by viruses for the sake of promoting the different steps of their replication cycles.
Keywords: cell membranes; electron microscopy; intracellular organelles; membrane rearrangements; nuclear envelope; peripheral endoplasmic reticulum (ER); vesicles; viral replication; virion assembly; virus-host interactions.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

