Serum Levels of Toxic AGEs (TAGE) May Be a Promising Novel Biomarker for the Onset/Progression of Lifestyle-Related Diseases
- PMID: 27338481
- PMCID: PMC4931418
- DOI: 10.3390/diagnostics6020023
Serum Levels of Toxic AGEs (TAGE) May Be a Promising Novel Biomarker for the Onset/Progression of Lifestyle-Related Diseases
Abstract
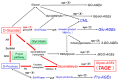
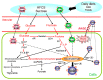
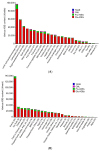
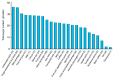
Advanced glycation end-products (AGEs) generated with aging or in the presence of diabetes mellitus, particularly AGEs derived from the glucose/fructose metabolism intermediate glyceraldehyde (Glycer-AGEs; termed toxic AGEs (TAGE)), were recently shown to be closely involved in the onset/progression of diabetic vascular complications via the receptor for AGEs (RAGE). TAGE also contribute to various diseases, such as cardiovascular disease; nonalcoholic steatohepatitis; cancer; Alzheimer's disease, and; infertility. This suggests the necessity of minimizing the influence of the TAGE-RAGE axis in order to prevent the onset/progression of lifestyle-related diseases (LSRD) and establish therapeutic strategies. Changes in serum TAGE levels are closely associated with LSRD related to overeating, a lack of exercise, or excessive ingestion of sugars/dietary AGEs. We also showed that serum TAGE levels, but not those of hemoglobin A1c, glucose-derived AGEs, or Nε-(carboxymethyl)lysine, have potential as a biomarker for predicting the progression of atherosclerosis and future cardiovascular events. We herein introduce the usefulness of serum TAGE levels as a biomarker for the prevention/early diagnosis of LSRD and the evaluation of the efficacy of treatments; we discuss whether dietary AGE/sugar intake restrictions reduce the generation/accumulation of TAGE, thereby preventing the onset/progression of LSRD.
Keywords: Alzheimer’s disease (AD); advanced glycation end-products (AGEs); biomarker; cancer; cardiovascular disease (CVD); infertility; lifestyle-related diseases (LSRD); nonalcoholic steatohepatitis (NASH); receptor for AGEs (RAGE); toxic AGEs (TAGE).
Figures
References
-
- International Diabetes Federation IDF Diabetes Atlas. 7th ed. 2015. [(accessed on 27 January 2015)]. Available online: http://www.diabetesatlas.org.
-
- Bucala R., Cerami A. Advanced glycosylation: Chemistry, biology, and implications for diabetes and aging. Adv. Pharmacol. 1992;23:1–34. - PubMed
-
- Vlassara H., Bucala R., Striker L. Pathogenic effects of advanced glycosylation: Biochemical, biologic, and clinical implications for diabetes and aging. Lab. Investig. 1994;70:138–151. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

